Prunus Pollen Cryopreservation

Carolyn DeBuse, USDA-ARS National Clonal Germplasm Repository for Tree Fruit, Nut Crops, and Grapes, One Shields Ave, University of California, Davis, California 95616.
Ashley N. Shepherd, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521.
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
Outline
- Prunus in the NPGS
- Collecting flowers
- Collecting pollen and anthers
- Moisture equilibration, packaging, and cryopreservation
- Retrieval, rehydration, and germination
- References
- Additional information
- Acknowledgments
1. Prunus in the National Plant Germplasm System (NPGS)
The genus Prunus includes several important fruit and nut crops including cherries, plums, peaches, nectarines, apricots, and almonds. In the USDA National Plant Germplasm System, Prunus collections are maintained in genebank unit locations in Davis, California and Geneva, New York. Some published work describes the preservation of Prunus pollen at 4, 0, -20, and -80 °C (Martínez-Gómez et al., 2002). Pollen preservation at liquid nitrogen temperatures has helped secure field collections of Prunus at the National Clonal Germplasm Repository for Tree Fruit & Nut Crops & Grapes in Davis. This chapter describes the process of collecting Prunus flowers from field-grown trees and then sifting to separate stamens and pollen from the petals, pistil, and other flower parts. After drying, sifted materials are placed into tubes and shipped to the National Laboratory for Genetic Resources Preservation (NLGRP) in Fort Collins, Colorado for further processing and long-term storage in liquid nitrogen.
2. Collecting flowers
During bloom, Prunus flowers progress through the following stages: 1) swollen dormant bud, 2) pink bud, 3) extended petals (popcorn), 4) unfurling petals, 5) fully open, 6) flattened petals, 7) abscised petals (Yi et al., 2006). For pollen collection, flowers are collected at the popcorn stage of bloom, where the petals are still firmly closed, but are swelling. Flowers are individually collected by hand and placed into a lunch-size paper bag, as demonstrated in the video below.
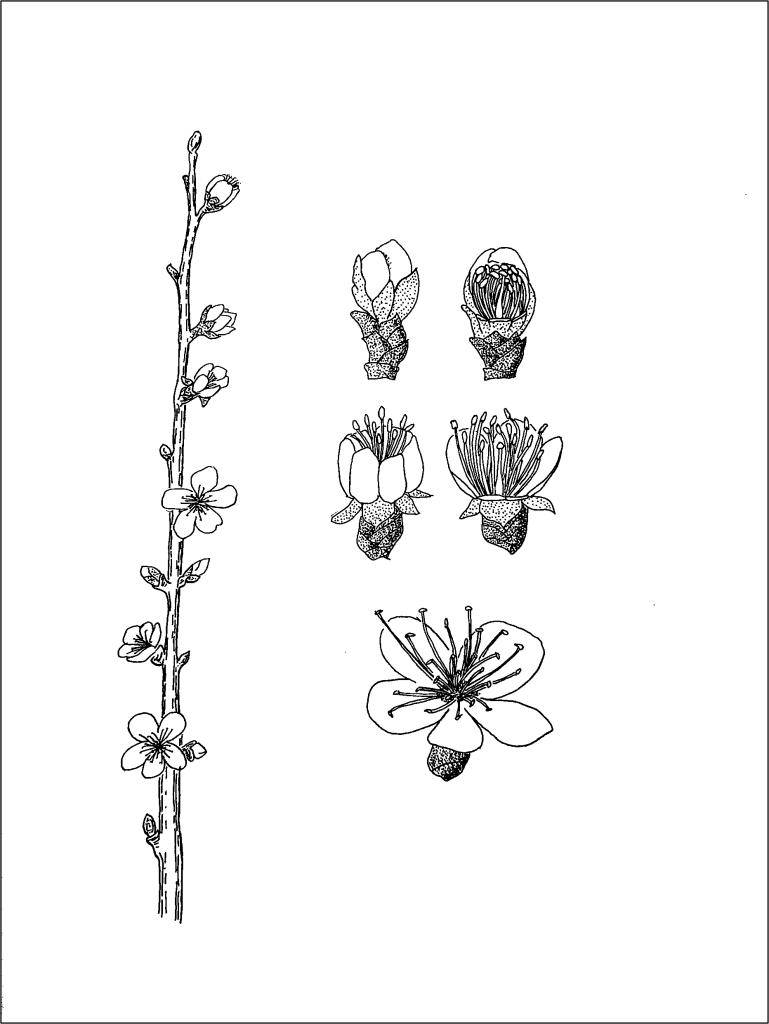

Harvested flowers are stored in a cooler for transport from the field to laboratory, taking care to protect the flowers from high temperatures and moisture. The number of flowers that need to be collected depends on the species and the specific accession. The weight of the collected fresh flowers can be measured to help determine if more or fewer flowers should be collected for future reference, after pollen yield is determined.

3. Collecting pollen and anthers
Anthers are removed from the flowers by rubbing the flowers over a screen by hand. The screen is a 1/8 inch mesh mounted on a 12 inch frame. The larger flower parts stay above the mesh and the anthers fall through onto a large piece of white paper. All larger flower parts are discarded. The sifted anthers are then shaken through a kitchen strainer screen onto a clean piece of white paper with the edges turned upwards. These processes are shown in the video below.
Anthers are dried overnight on the laboratory bench (8-12 hours) at room temperature (18-20 °C) and ambient humidity. The drying process releases pollen from the anthers.

A funnel is serves to collect the resulting pollen and anthers into a tube for shipment. Tubes are immediately shipped overnight to NLGRP in an insulated package with an ice pack. Ideally, 10-15 mL of dried Prunus anthers and pollen are sent to NLGRP. This will result in 15 vials containing 0.5 to 1 mL of anthers and pollen for long-term storage.
The screen, strainer, and hands are cleaned with isopropyl alcohol and then dried between flower samples. This will kill any residual pollen before the next use.
4. moisture equilibration, packaging, and cryopreservation
Once received at NLGRP, a small volume of anthers/pollen is sacrificed for moisture content calculations. The remaining Prunus anthers/pollen are placed into an open Petri dish that is then set in a desiccation chamber overnight. The environment in the glass desiccation chamber has an approximate relative humidity of 46% because it is adjusted over a saturated salt solution of calcium nitrate. Information on making a saturated salt slurry as well as establishing moisture content are described in an earlier chapter.

After overnight moisture equilibration, moisture content is calculated from another small aliquot of anthers/pollen. Average moisture contents for Prunus pollen/anthers cryopreserved at NLGRP are shown in the table below, expressed on a fresh weight basis (fwb).
| Average moisture content (fwb) | Range of moisture content (fwb) | |
| Pollen just received at NLGRP | 0.124 g H2O/g | 0.057-0.212 g H2O/g |
| Pollen after Ca(NO3)2 equilibration | 0.088 g H2O/g | 0.042-0.142 g H2O/g |
Pollen/anthers are placed in fifteen 1.2 mL cryovials in 0.5 mL aliquots, which are then loaded onto canes and stored in cryocanisters. Canisters are placed directly into a cryotank and stored in the vapor phase of liquid nitrogen. This allows for 15 distributions of pollen for crosses, one vial per distribution.
5. retrieval, rehydration, and germination
To assess viability of cryopreserved material, an aliquot of pollen is taken from one cryovial. The vial is carefully removed from the vapor phase of liquid nitrogen, ensuring the remaining vials do not warm. The cryovial is set at room temperature (~22 °C) to warm for 10 minutes.
After the vial warms to room temperature, some of the pollen/anther mixture is placed in a small, empty Petri dish; the amount needed varies greatly depending on how much pollen is present. To rehydrate the material, the small Petri dishes should be placed above a water-saturated paper towel within a larger Petri dish. The larger Petri dish is covered with its lid and kept for 2 hours at room temperature in the dark.
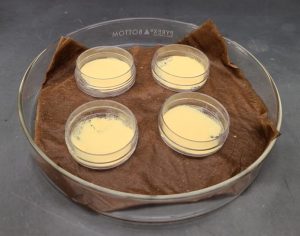
Prunus pollen viability is assessed through in vitro germination. A 20% sucrose germination medium (20% sucrose + 0.01% boric acid + 0.03% calcium nitrate + 0.02% magnesium sulfate + 0.01% potassium nitrate + 2% agarose) is dispensed into 10 x 35 mm plastic Petri dishes (Marquard, 1992). After rehydrating for 2 hours, the pollen/anther mixture is poured onto the semisolid germination medium. Anthers are then knocked off the medium and the plate with pollen is placed in the dark at room temperature for about 18-24 hours. The process of applying pollen to the medium is shown in the video below.
The pollen grains on the medium are observed at 200x magnification with a compound microscope. Viability percentages are calculated by counting 100 pollen grains on each of three plates for each cryopreserved inventory. Pollen tubes that are longer than the diameter of the pollen grain are considered germinated. Inventories are kept in long-term preservation when the average viability of the three plates is greater than 5%.

6. References
Marquard RD. 1992. Pollen Tube Growth in Carya and Temporal Influence of Pollen Deposition on Fertilization Success in Pecan. Journal of American Society of Horticulture Science 117:328-331. DOI: 10.21273/JASHS.117.2.328
Martínez-Gómez P, Gradziel TM, Ortega E, Dicenta F. 2002. Low temperature storage of almond pollen. HortScience 37:691-692. DOI: 10.21273/HORTSCI.37.4.691
Yi W, Law SE, McCoy D, Wetzstein HY. 2006. Stigma development and receptivity in almond (Prunus dulcis). Annals of Botany 97:57-63. DOI: 10.1093/aob/mcj013
7. Additional information
Download the Prunus pollen cryopreservation procedure used at the NLGRP.
Download the Prunus pollen recovery procedure used at the NLGRP.
8. Acknowledgments
Chapter citation: DeBuse C, Shepherd AN, Volk GM. 2025. Prunus pollen cryopreservation. In: Volk GM, Chen KY (Eds.) Pollen Preservation. Fort Collins, Colorado: Colorado State University. Date accessed. Available from: https://colostate.pressbooks.pub/pollenpreservation/chapter/prunus-pollen-cryopreservation/
This training module was made possible by:
Content providers: Carolyn DeBuse, Ashley N. Shepherd, Gayle M. Volk
Videographers: Carolyn DeBuse, Emma Balunek, Remi Bonnart, Katheryn Y. Chen
This project was funded by the USDA-ARS and by the USDA-NIFA Higher Education Challenge Program grant 2020-70003-30930. USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.

