Pistachio Pollen Cryopreservation
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
Jenny Smith, USDA-ARS National Clonal Germplasm Repository for Tree Fruit, Nut Crops, and Grapes, One Shields Ave, University of California, Davis, California 95616.
Ashley N. Shepherd, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521.
Outline
- Pistachio in the NPGS
- Collecting catkins
- Collecting pollen from catkins
- Moisture equilibration, packaging, and cryopreservation
- Retrieval, rehydration, and germination
- References
- Additional information
- Acknowledgments
1. Pistachio in the National Plant Germplasm System (NPGS)
The NPGS pistachio collection is maintained at the USDA National Clonal Repository for Tree Fruit, Nut Crops and Grapes in Davis, California (Preece et al., 2021). The collection maintains more than 200 accessions representing 12 Pistaceae species that are maintained as trees in the field. Pollen is transferred by wind from male pistachio trees to female pistachio trees (Ferguson et al., 2005). At this time, none of the NPGS pistachio trees are backed up at the USDA-ARS National Laboratory for Genetic Resources Preservation (NLGRP) in Fort Collins, Colorado as seeds (nuts), dormant buds or shoot tips. Each year, pollen is collected and cryopreserved from the male trees in the Davis collection.
2. Collecting catkins
In mid-spring, pollen is collected from the catkins of field-grown pistachio trees at the Wolfskill Experimental Orchard where the collections of the USDA Clonal Germplasm Repository for Tree Fruit, Nut Crops, and Grapes in Davis, California are maintained. Staff regularly monitor trees and catkins are collected just as they begin to dehisce pollen. Harvested catkins are placed into labeled paper bags (lunch bag size, approximately 2500-3000 cubic centimeters in volume), then brought directly to the laboratory for processing. This will yield approximately 50 mL of pollen.

3. Collecting pollen from catkins
At the lab in Davis, the catkins are spread out in a single layer inside labeled trays. These are allowed to dry at room temperature (~22 °C) for 24-48 hours. Released pollen will become visible at the bottom of trays.

After the catkins have shed pollen, a clean paintbrush can help knock extra pollen off of the catkins. Catkins are placed into a coarse strainer to separate the pollen into a large container. Collect loose pollen clinging to the trays with the paintbrush. After initial sifting, insects, plant tissues, or other large debris will be present. To extract just the pollen, carefully pour contents from the large container into a fine mesh sieve. Sift the pollen onto clean paper. This process of capturing pollen from pistachio catkins is shown in the video below.
Sieved pollen is decanted from the paper into clean 50 mL tubes. These must be well-labeled, and stored in refrigeration (ideally less than one day) until they can be packaged on ice and shipped to the National Laboratory for Generic Resources Preservation (NLGRP) in Fort Collins, Colorado.
All materials must be sanitized with alcohol between accessions to prevent any cross contamination.
4. Moisture equilibration, packaging, and cryopreservation

When received at NLGRP, a small sample of each pollen accession is weighed (fresh weight), then dried for several days at 90 °C and re-weighed (dry weight). The moisture content of all remaining pistachio pollen is then adjusted by placing it into a Petri dish. The dish is then placed over saturated salts of calcium nitrate (46% relative humidity) at room temperature overnight. Saturated salts of calcium nitrate are made by pouring the salt solution into a Petri dish and then adding water to make a slurry. After moisture adjustment, another small sample is weighed, then placed into an oven to later determine dry weight. More information on saturated salt solutions, moisture equilibration, and moisture calculations are described in an earlier chapter.
After moisture adjustment, pollen is placed in fifteen 2 mL cryovials, which are then loaded onto canes and stored in cryocanisters in the vapor phase of liquid nitrogen in a cryotank. This provides 15 distributions of pollen for crosses, 2 mL per distribution. The video below shows the process of moisture adjusting, packaging, and cryopreserving pollen (note that the video shows walnut pollen, which follows the same process as pistachio pollen).
5. Retrieval, rehydration, and germination
To determine viability of the stored material, a technician carefully removes one cryovial containing pollen from the vapor phase of liquid nitrogen, ensuring the remaining vials do not warm. In some cases, a very small amount of pollen can be taken from a vial while keeping it cold; pollen remaining in the vial is then immediately returned to the vapor phase of liquid nitrogen for continued storage. The technician allows the cryovial (or pollen aliquot) to warm at room temperature (~22 °C) for 10 minutes.
After pollen warms to room temperature, the technician scoops a very small amount from the vial (<15 mg needed) and places it into a small Petri dish. To rehydrate the pollen, the small Petri dishes are placed in a high-humidity environment. At NLGRP, this is achieved by placing them on a water-saturated paper towel within a larger Petri dish with a lid, then letting them rehydrate for 2 hours at room temperature in the dark.
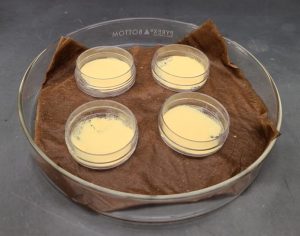
Pistachio pollen viability is assessed via in vitro germination. Germination medium (15% sucrose + 0.01% boric acid + 0.03% calcium nitrate + 0.02% magnesium sulfate + 0.01% potassium nitrate + 2% agarose) is dispensed into 10×35 mm plastic Petri dishes (Marquard, 1992). The technician applies a small, soft bristled paint brush to carefully pick up a dusting of pollen with just the tips of the bristles. With a very gentle tap of the brush, they lightly dust the surface of the medium, ensuring that the pollen is evenly spread. The lid is placed back onto the Petri dish, which is placed into the dark at room temperature for about 18-24 hours. The technique for applying pollen to the germination medium is demonstrated in the below video (note that the video shows date palm pollen, which follows the same process as pistachio pollen).
The pollen grains on the medium are observed at 200x magnification. Viability percentages are calculated by counting 100 pollen grains on each of three plates for each cryopreserved inventory. Pollen tubes longer than the diameter of the pollen grain are considered germinated. Inventories are kept in long-term preservation when the average viability of the three plates is greater than 5%.
6. References
Ferguson L, Polito V, Kallsen C. 2005. The pistachio tree; botany and physiology and factors that affect yield. Pistachio production manual, 4th ed. Davis, CA, USA, University of California Fruit & Nut Research Information Center, 31-39.
Marquard RD. 1992. Pollen Tube Growth in Carya and Temporal Influence of Pollen Deposition on Fertilization Success in Pecan. Journal of American Society of Horticulture Science 117:328-331. DOI: 10.21273/JASHS.117.2.328
Preece JE, Balunek E, Volk GM. 2021. Pistachio collection. In: Volk GM, Preece JE (Eds.) Field tour of the USDA National Clonal Germplasm Repository for Tree Fruit, Nut Crops, and Grapes in Davis, California. Fort Collins, Colorado: Colorado State University. Accessed January 2025. Available from: https://colostate.pressbooks.pub/davisrepositoryfieldtour/chapter/pistachios/
7. Additional information
Download the pistachio pollen cryopreservation procedure used at the NLGRP.
Download the pistachio pollen recovery procedure used at the NLGRP.
8. Acknowledgments
Chapter citation: Volk GM, Smith J, Shepherd AN. 2025. Pistachio pollen cryopreservation. In: Volk GM, Chen KY (Eds.) Pollen Preservation. Fort Collins, Colorado: Colorado State University. Date accessed. Available from https://colostate.pressbooks.pub/pollenpreservation/chapter/pistachio-pollen-cryopreservation/
This training module was made possible by:
Content providers: Gayle M. Volk, Jenny Smith, Ashley N. Shepherd
Videographers: Jenny Smith, Emma Balunek, Remi Bonnart, Katheryn Y. Chen
This project was funded by the USDA-ARS and by the USDA-NIFA Higher Education Challenge Program grant 2020-70003-30930. USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.


