Date Palm Pollen Cryopreservation
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
Ashley N. Shepherd, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521.
Annie Carolina Araújo de Oliveira, Federal University of Sergipe, Marechal Rondon Ave., São Cristóvão, SE 49100‑000, Brazil.
Robert R. Krueger, USDA-ARS National Clonal Repository for Citrus and Dates, 1060 Martin Luther King Blvd., Riverside, California 92507.
The purpose of this training is to demonstrate the process of collecting, preparing, preserving, and conducting viability assays of date palm pollen for long-term storage. This training is designed for scientists and technical staff with horticultural, cryopreservation, or plant tissue culture experience.
Outline
- Date palm in the NPGS
- Collecting spathes
- Collecting pollen from spathes
- Moisture equilibration, packaging, and cryopreservation
- Retrieval, rehydration, and germination
- References
- Additional information
- Acknowledgments
1. Date palm in the National Plant Germplasm System (NPGS)

The USDA-ARS National Clonal Germplasm Repository for Citrus & Dates (NCGRCD) is responsible for maintaining the primary collection of date palm in the National Plant Germplasm System. The physical date palm plants grow in Thermal, California at the University of California at Riverside Coachella Valley Agricultural Research Station (CVARS). The collection comprises of more than 130 accessions, the majority of which are Phoenix dactylifera L.; because the trees are dioecious, these accessions are represented by a combination of male and female mature plants. As of 2023, pollen has been collected and cryopreserved from all the male trees in the collection at the USDA-ARS National Laboratory for Genetic Resources Preservation (NLGRP) via the cryopreservation methods published by de Oliveira et al. (2021; 2023).
2. Collecting spathes

Date palm inflorescences grow in large structures known as spathes, which form in the tree crowns during spring. As the anthers reach maturity, male spathes change color and soften slightly before cracking open and quickly releasing pollen. To prevent or limit splitting and premature pollen release, NCGRCD staff tie the spathes and monitor their development closely. Technicians harvest the spathes as soon as they see signs of maturity, using an aerial work platform as shown in the video below.
3. Collecting pollen from spathes
If necessary, the spathe is cut open with loppers to release the mature anthers. The anthers are then placed in wardrobe boxes lined with white blotter paper. As the anthers dry, pollen is released onto the blotter paper.


After 2-3 weeks, technicians shake out any remaining pollen from the anthers, then sift it to remove any floral parts, debris, and insects. Date palm pollen sieves at CVARS are constructed with a 2-3 mm mesh (U.S. standard mesh size 7-12), although the small size of date palm pollen (mean length of 17-27 µm and mean width of 10-16 µm) would allow for a finer sieve if needed. At CVARS the robust inflorescences typically produce 200-300 mL of pollen each; therefore, many accessions require only a single spathe to meet conservation goals. After the pollen is released and sifted, it is packaged into vials or sealed containers, carefully labeled, and shipped to the NLGRP in Fort Collins, Colorado for long-term preservation.

4. Moisture equilibration, packaging, and cryopreservation
Upon receipt, a small sample of each pollen accession is weighed (fresh weight), then dried for several days at 90 °C and re-weighed (dry weight). The moisture content of the date palm pollen can be equilibrated by placing it in glass desiccators over saturated salts of calcium nitrate (relative humidity 46%) at room temperature (~22 °C) and letting it sit overnight. Alternatively, pollen can be dried in a moisture-controlled room overnight; at NLGRP this occurs in a cold room set at 5 °C and 23% relative humidity. After moisture adjustment, another small sample is weighed, then placed into an oven to later determine dry weight. Information on preparing saturated salt solutions, moisture equilibrating, and moisture content calculation is shown in an earlier chapter.

After remaining in the sealed desiccator overnight, date palm pollen is aliquoted into fifteen 4 mL polypropylene vials. Four milliliter quantities were selected to ensure adequate pollen is available for crosses when a vial of pollen is distributed. Vials are placed onto aluminum canes, covered by protective sleeves, and organized into cryocanisters. Canisters are then placed directly into the vapor phase of liquid nitrogen in a cryotank. The packaging and freezing process is shown in the video below.
5. Retrieval, rehydration, and germination
To determine viability of the stored pollen, a technician carefully removes one cryovial containing pollen from the vapor phase of liquid nitrogen, ensuring the remaining vials do not warm. In some cases, a very small amount of pollen can be taken from a vial while keeping it cold; pollen remaining in the vial is then immediately returned to the vapor phase of liquid nitrogen for continued storage. The cryovial (or pollen aliquot) is warmed at room temperature (~22 °C) for 10 minutes.
After pollen warms to room temperature, the technician scoops a very small amount from the vial (<15 mg) and places it into a small Petri dish. To rehydrate the pollen, the small Petri dishes are placed in a high-humidity environment. At NLGRP, this is achieved by placing them on a water-saturated paper towel within a larger Petri dish with a lid, then rehydrating for 2 hours at room temperature in the dark.
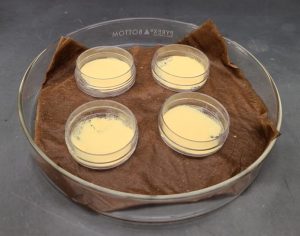
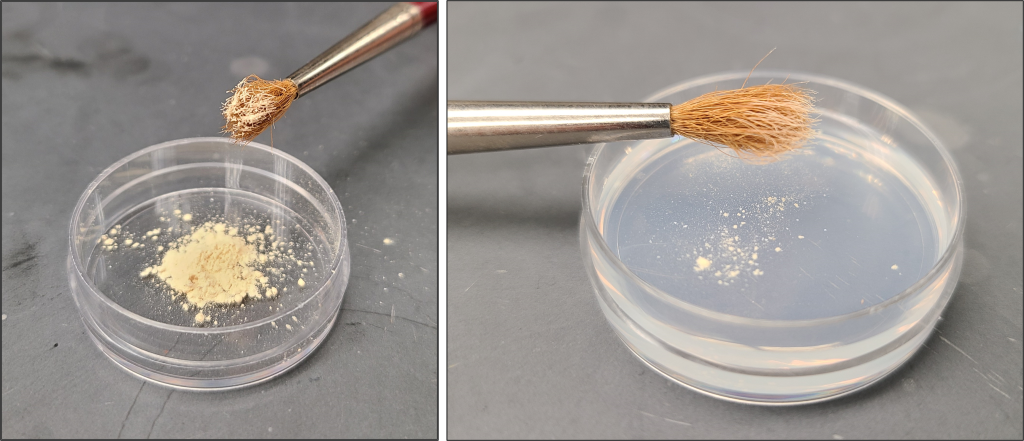
Date palm pollen viability is assessed through in vitro germination. Germination medium (15% sucrose + 0.01% boric acid + 0.03% calcium nitrate + 0.02% magnesium sulfate + 0.01% potassium nitrate + 2% agarose) is dispensed into 10 x 35 mm plastic Petri dishes (Marquard, 1992). The technician applies a small, soft bristled paint brush to carefully pick up a dusting of pollen with just the tips of the bristles. With a very gentle tap of the brush, they lightly dust the surface of the medium, ensuring that the pollen is evenly spread. The lid is placed back onto the Petri dish, which is placed into the dark at room temperature for about 18-24 hours. The technique for applying pollen to the germination medium is demonstrated in the below video.
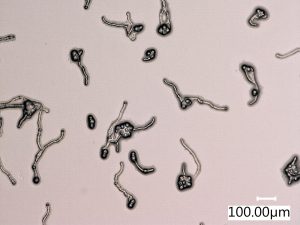
The pollen grains on the medium are observed at 200x magnification. Viability percentages are calculated by counting 100 pollen grains on each of three plates for each cryopreserved inventory. Pollen tubes longer than the diameter of the pollen grain are considered germinated. Inventories are kept in long-term preservation when the average viability of the three plates is greater than 5%.
6. References
de Oliveira ACA, da Silva AS, Polek ML, Krueger RR, Shepherd A, Volk GM. 2023. Methods for cryopreserving date palm pollen. In: Rajasekharan PE, Rohini MR (eds.), PollenCryopreservationProtocols, Springer Protocols Handbooks. DOI: 10.1007/978-1-0716-2843-0_49
de Oliveira, ACA, da Silva AS, Polek ML, Krueger RR, Shepherd A, Volk GM. 2021. Optimization of in vitro germination and cryopreservation conditions for preserving date palm pollen in the USDA National Plant Germplasm System. Plant Cell, Tissue and Organ Culture. 144:223-232. DOI: 10.1007/s11240-020-01907-1
Marquard RD. 1992. Pollen Tube Growth in Carya and Temporal Influence of Pollen Deposition on Fertilization Success in Pecan. Journal of American Society of Horticulture Science 117:328-331. DOI: 10.21273/JASHS.117.2.328
7. Additional information
Download the date palm pollen cryopreservation procedure used at the NLGRP (this version highlights the cold room technique for moisture equilibration).
Download the date palm pollen recovery procedure used at the NLGRP.
8. Acknowledgments
Chapter citation: Volk GM, Shepherd AN, de Oliveira ACA, Krueger RR. 2025. Date palm pollen cryopreservation. In: Volk GM, Chen KY (Eds.) Pollen Preservation. Date accessed. Available from https://colostate.pressbooks.pub/pollenpreservation/chapter/date-palm-pollen-cryopreservation/
This training module was made possible by:
Content providers: Gayle M. Volk, Ashley N. Shepherd, Annie Carolina Araújo de Oliveira, Robert R. Krueger
Videographers: Ashley N. Shepherd, Remi Bonnart, Katheryn Y. Chen
This project was funded by the USDA-ARS and by the USDA-NIFA Higher Education Challenge Program grant 2020-70003-30930. USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.


