Maintaining and ensuring plant health in seed germplasm collections: an applied plant pathology perspective

Anna L. Testen, Research Plant Pathologist, USDA-ARS, Application Technology Research Unit, Wooster, OH Anna.Testen@usda.gov
Previous Position: Plant Pathologist, USDA-ARS Plant Introduction Research Unit, Ames, IA
OUTLINE
- Introduction
- Photosanitary concepts relating to germplasm exchange
- Diagnosing and managing diseases in the context of germplasm collections
- Meeting phytosanitary requirements through field inspections, laboratory testing, and seed treatments
- Research to improve plant health in germplasm collections
- References
- Additional information
- Acknowledgments
1. Introduction
Preserving plant germplasm is critical to weathering emerging challenges from pests and diseases, habitat loss, and climate change. Plant diseases, caused by bacteria, viruses and viroids, fungi, oomycetes, parasitic plants and nematodes, affect all plants. In order to ensure germplasm is viable and available to combat incoming plant disease challenges, plant pathologists work to maintain the health of germplasm collections, ensure compliance with phytosanitary regulations in international germplasm distributions, and develop methods to detect and manage seedborne and propagule-borne (e.g. tubers, cuttings, budwood) diseases. This chapter provides practical knowledge and resources describing how plant pathologists support and improve plant health in seedbanks and germplasm collections through disease diagnostics, disease management and seed health testing.
2. Phytosanitary Concepts Related to Germplasm Exchange
Understanding phytosanitary regulations is critical to enabling safe and uninterrupted plant germplasm distributions. An overview of key concepts and documents in phytosanitary issues is provided in this section. For a more detailed description of phytosanitary regulations related to germplasm exchange, please see the chapter “Phytosanitary and Regulatory Issues in the Movement of Plant Genetic Resources.”
Plant pathologists work with local, national (NPPO), and regional (RPPO) Plant Protection Organizations (PPOs) to ensure compliance with phytosanitary regulations. For example, the plant pathologist at the United States Department of Agriculture (USDA) Agricultural Research Service (ARS) North Central Regional Plant Introduction Station (NCRPIS) in Ames, Iowa, USA, works with the local PPO: the Iowa Department of Agriculture and Land Stewardship (IDALS), the NPPO: USDA Animal and Plant Health Inspection Service (APHIS), and the RPPO: North American Plant Protection Organization (NAPPO).
Import permits and phytosanitary certificates
Several documents enable international movement of germplasm. Two main documents that plant health practitioners must understand are the import permit and the phytosanitary certificate. An import permit is provided by an importing country and lists the conditions that must be met by the germplasm exporter to allow germplasm into the importing country. For example, an import permit may list conditions such as germplasm “must be free of soil and weed seeds” or free from specific pests. Import permit conditions that relate to specific pests usually describe how the exporting country may meet these conditions, such as by field inspections or specific laboratory testing. The Phytosanitary Export Database, maintained by USDA-APHIS, provides export conditions for specific countries for seeds originating in the United States. Seed origin is based on the country in which the seed was produced. A germplasm exporter must obtain a phytosanitary certificate from local or national PPOs to demonstrate the health of the germplasm and that all specific conditions listed in the import permit were met. Exporting countries confirm that they have fulfilled specific conditions listed in import permits in a phytosanitary certificate section known as additional declarations. Additional declarations must be made in a way that meets the conditions listed on the original import permit. For example, if an importing country requests that freedom from viruses be fulfilled by a field inspection of seed parent plants, the exporting country cannot make an additional declaration for freedom from viruses based on laboratory seed health testing. Not all importing countries require phytosanitary certificates for certain species of germplasm. For some crops or countries, import permits are not provided, but a phytosanitary certificate must be provided to ensure seed health.
International Standards for Phytosanitary Measures
International Standards for Phytosanitary Measures (ISPM) are developed by the Commission on Phytosanitary Measures as part of the International Plant Protection Convention (IPPC). These standards provide guidelines for globally important phytosanitary issues, ranging from wood packaging to phytosanitary certificates. Plant pathologists working with germplasm collections should be familiar with relevant ISPM documents including ISPM 05: Glossary of phytosanitary terms, ISPM 12: Phytosanitary certificates, ISPM 23: Guidelines for inspection, ISPM 31: Methodologies for sampling of consignments, and ISPM 38 International movement of seeds. These relevant ISPM help plant pathologists in germplasm collections develop guidelines to improve distribution of germplasm in their collections and work with phytosanitary officials across the world.
New germplasm enters collections from both domestic and foreign sites, yet germplasm from any location should be handled carefully to ensure that new pests or pathogens are not being introduced. For germplasm from foreign locations, NPPOs may handle inspection and quarantine of the propagative material. When new germplasm is received into a collection, the pathologist should carefully inspect the seeds and assess the risk of introducing new pathogens to the collection. Supporting phytosanitary records, such as phytosanitary certificates with field inspection or seed testing results, are sometimes provided with the material and can help in assessing risks. These records can suggest additional testing that may need to be conducted prior to propagation of these materials. Initial propagations of new germplasm should be conducted in greenhouses or an isolated field to reduce risks of disease spread. Plants should be observed routinely for signs of diseases.
3. Diagnosing and Managing Diseases in the Context of Germplasm Collections
Plant disease diagnostics
Maintaining plant health during germplasm regeneration (propagation) is essential to reducing the risk of seedborne or propagule-borne pathogen spread via future germplasm distributions. Plant health is rooted in good horticultural and agronomic production practices, but diseases still occur even under the best conditions. Reducing the impacts of plant diseases hinges on proper disease diagnosis.
Accurate disease diagnosis is necessary to identify the correct management strategies for a given disease, but disease diagnostics in germplasm production can be difficult. One of the difficulties of diagnosing diseases within a germplasm collection is the sheer number of different species and accessions within single species. Little is known about the common diseases of some taxa, while even well characterized crops may have accessions with atypical disease symptoms. Another challenge to adequate diagnostics in germplasm collections is if curators are unwilling or unable to sacrifice valuable seed parent plants for diagnosis, so non-destructive diagnosis should be attempted. Even with these challenges, diagnostics within a germplasm collection is a rewarding endeavor that can lead to improved plant health in the collection and an improved understanding of diseases of less well-known taxa.
To start, a plant pathologist should assemble a diagnostics toolbox of resources to help with proper identification of plant diseases. The most important tools in this toolbox are a hand lens and pocketknife (see Table 1 for the roles of these tools). Other items in the diagnostics toolbox include plastic bags, a spade or small shovel, pruners, permanent marker, notebook and pen, tape, ruler or measuring tape, flags and flagging tape, 70% ethanol, water in squirt bottle, paper towels, and an insulated cooler. This toolbox should also include a mini reference library of disease factsheets and disease compendia to aid in visual identification of diseases. With these assembled tools, a plant pathologist is able to tackle field-based diagnostics.
Table 1. Items in a field disease diagnostics kit and their purposes.
| Item | Purpose |
| Hand lens | To observe magnified symptoms, useful for seeing fungal structures within lesions |
| Pocket knife | To cut open stems or take samples from a plant |
| Spade or small shovel | To dig up roots for observation |
| Pruners | To take samples from a plant |
| Permanent marker | To label samples or flags |
| Notebook and pen | To take notes on samples or plant history |
| Ruler or measuring tape | To measure symptoms or demarcate a location in the field |
| Flags/flagging tape | To mark important areas or plants in a field |
| 70% ethanol | To preserve samples or sterilize tools and boots between fields |
| Water in a squirt bottle | To remove soil and debris from samples |
| Plastic bags | For sample storage |
| Paper towels | To wrap delicate samples, keep samples moist, or dry samples |
| Insulated cooler | To preserve samples between the field and lab |
| Reference materials | For visual comparison of disease symptoms and information on pathogens and disease cycles |
The first step of diagnostics is to identify the host and to recognize the appearance of a healthy host plant. By identifying the host species and sometimes focusing on a specific genetic background, a plant pathologist can identify which diseases are most likely and eliminate diseases that do not occur for that host. Due to diversity within germplasm collections, some accessions may display atypical disease reactions or may appear infected when healthy (Figure 1). Next, the pathologist should gather background information about the affected plants, such as field and soil conditions, environmental conditions prior to appearance of symptoms, distribution of the symptoms in the field/greenhouse, and pesticides or fertilizers applied to or near the affected plants. This background information can suggest if the cause is biotic or abiotic and is especially important for identifying potential abiotic disorders, such as heat damage, cold damage, or pesticide drift. More information on abiotic disorders is available from the American Phytopathological Society (APS). The pathologist should note symptom distribution on the plants. If symptoms clearly match a specific disease, the pathologist can make a diagnosis in the field with good confidence. However, field diagnoses are not always possible as many diseases present similar symptoms, and pathologists may need to bring samples to the lab to identify the disease.

Figure 1. Lesion mimic mutants in maize. Although the plants appear infected, these spots are naturally caused by host genetics, not by a pathogen. Photo Credit: Dr. Charles Block, Iowa State University.
In the lab, pathologists often make an initial check for bacterial streaming or fungal structures under the microscope. This can help in making a positive disease identification or eliminate possible diseases if pathogen signs are not present under the microscope. Another technique that plant pathologists use is incubating plant material in a humid environment, a moist chamber, to encourage the growth of fungal and oomycete structures that would later be observed under the microscope. In culture-based diagnostics methods, pathologists isolate microbes from infected plant tissue on agar-based media to identify the pathogen causing the disease. For diseases caused by viruses, molecular testing, such as reverse transcriptase polymerase chain reaction (RT-PCR), or serological methods, such as enzyme linked immunosorbent assay (ELISA), must be used to identify the causal virus. Molecular and serological methods can also be used to confirm specific bacterial, fungal and oomycete diseases. Sequencing approaches, including high-throughput sequencing (HTS), can also be used to identify potential pathogens. These methods will be described in more detail in the seed health testing section of this chapter.
A plant pathologist should keep notes on the occurrence of diseases and their hosts and when they occur as this can help inform future diagnoses. These notes can be used to identify which diseases occur most commonly in the germplasm collection and when (time of year of phenological stage) to scout for them. This documentation can be used to create documents to help curators and staff identify these diseases in their plants. For example, these records were used to create a series of plant disease identification and management guides specific to key diseases of specific taxa at the North Central Regional Plant Introduction Station (Figure 2). These guides are consulted by staff and students at the station to learn how to scout for and identify common diseases. These cards also describe management options feasible for use at the station. These cards aid plant health overall because diseases can be detected earlier by more informed observers, rather than just the plant pathologist on duty.
More information on plant disease diagnostics is available from APS.

Figure 2. Examples of entries in disease identification and management guides developed for use by program staff at the North Central Regional Plant Introduction Station. Cards provide basic information on the disease name and causal agent, key symptoms and scouting guidelines, and management guidelines for the diseases. Diseases listed in each guide are limited to those most important in the seedbanks’ production region and diseases of major phytosanitary concern.
Plant disease management
In plant disease management, an ounce of prevention is worth a pound of cure. Plant pathologists should encourage good crop production practices that reduce plant stress and promote development of healthy plants. These practices are numerous. The most important decision is site selection and selection of sites best suited to the production of specific taxa. This is not always possible, especially when growing certain taxa far from their native range. At the very least, sites should have good drainage and good air movement. Crops should be rotated to avoid buildup of soilborne pathogens, while avoiding planting taxa from the same family in the same location year after year. During plant growth, plants should receive adequate water and fertilization. Over- or under-fertilization can predispose plants to certain diseases, while over-watering can predispose plants to root rots. Practices to increase and maintain soil health should be promoted such as cover cropping, building soil carbon through incorporation of organic amendments, reduction of machinery passes across fields, and reduced tillage. Soil health within plots should be monitored and documented and can be done with low cost options such as the USDA NCRS soil quality test kit. Soil health maps can be generated through participatory activities, such as soil health mapping, and generated and shared using programs, such as Google Earth (Figure 3).
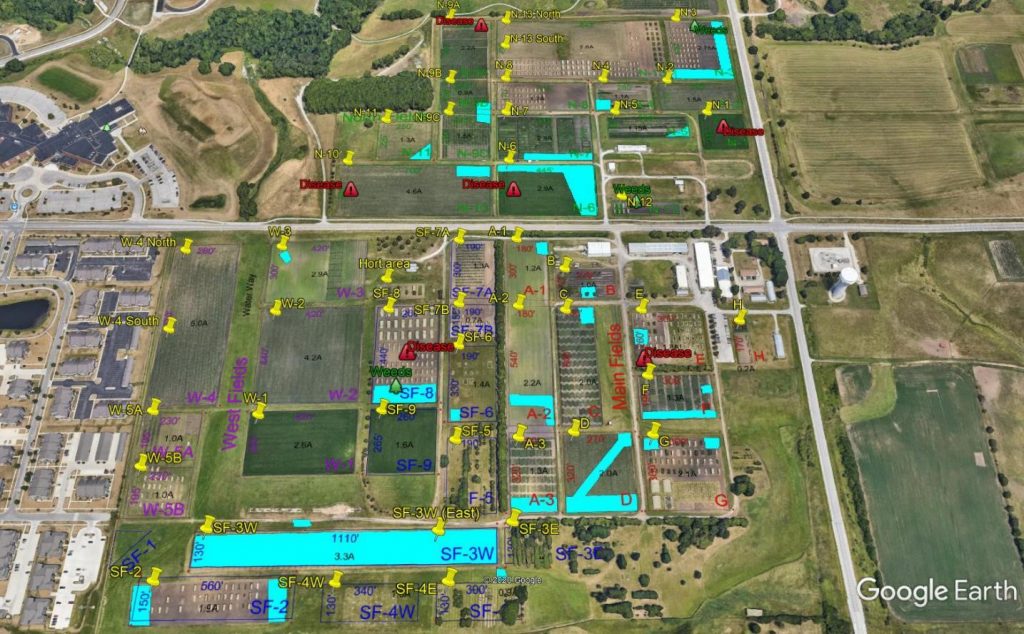
Figure 3. An example of a soil map of the North Central Regional Plant Introduction Station in Ames, Iowa generated in Google Earth. Blue areas were identified by unit staff as having drainage issues. Yellow pins can be clicked on to access soil testing results for a field, while red hazard icons or green trees can be clicked on for more information on soilborne disease or weed issues, respectively.
In the previous section on disease diagnostics, we discussed proper diagnostics of plant diseases in order to identify ideal disease management strategies. For example, if a bacterial disease is misdiagnosed as a fungal disease and a fungicide treatment is applied, that treatment will have no impact on the disease and represents wasted resources and unnecessary environmental impact. A detailed review of plant disease management is available from APS.
Five key principles and their related questions should be considered when conducting and developing disease management plans and these include:
- Scouting: Which diseases are present and at what level? What are the ideal times and conditions to scout for these diseases?
- Disease diagnostics: Which diseases are present so I can make ideal management decisions?
- Thresholds: When should disease management strategies be applied? Is this disease actually a problem in germplasm production?
- Management strategies: What are my disease management options for a given disease? Which management strategies are most feasible in the germplasm production system I am working in?
- Record keeping: Which management strategies were applied, and did they work?
The types of applicable disease management strategies can be classified as avoidance, exclusion, protection, eradication, and resistance.
- Avoidance refers to strategies designed to avoid locations or conditions conducive to disease. Examples of avoidance is to avoid planting in low lying areas of a field for accessions susceptible to root rot or to delay planting until soil temperatures are warmer.
- Exclusion refers to preventing pathogen introduction to a system. An example of exclusion is the use of “clean” (pathogen-free) seeds or propagules.
- Protection refers to physically or chemically protecting plants from infection. Examples of protection include the use of protectant fungicides or growing plants in a high tunnel setting rather than an open field.
- Eradication refers to efforts to eliminate established pathogens. Examples include use of curative fungicides, seed treatments, or soil disinfestation methods, such as anaerobic soil disinfestation.
- Resistance refers to the use of disease resistant varieties, which is usually the foundation of any successful disease management program. However, given the diversity within germplasm collections, there is little one can do about accessions that lack disease resistance. Knowing whether accessions are susceptible can help determine alternative disease management strategies ahead of time.
When a plant pathologist is identifying the best disease management strategies to apply, they should distinguish between diseases of phytosanitary concern (highest priority), diseases not of phytosanitary concern but are detrimental to seed/propagule quality (middle priority), and diseases that are not of phytosanitary concern and do not affect seed quality (lowest priority). Diseases of phytosanitary concern are of highest priority as their presence will hinder germplasm distribution, and there is often zero tolerance for the presence of these diseases. Management approaches for diseases of phytosanitary concern are often implemented at the first sign of symptoms or even pre-emptively. Diseases of phytosanitary concern may require immediate destruction of parent plants so care should be taken to ensure that backup seedstocks are available. Diseases that affect seed and propagule quality should also be carefully managed to avoid reductions in seed and propagule longevity. Diseases that cause only aesthetic damage to accessions do not require management.
Emerging Diseases
New diseases emerge and become phytosanitary issues as cropping practices change, varieties with disease susceptible-genetic backgrounds are widely grown, and novel pathogens or strains develop or are introduced to a region. Plant pathologists working in seedbanks should work to keep up to date on the emerging issues of phytosanitary concern in the key crops in their collections. Practical ways to keep up to date with emerging diseases include joining crop-specific working groups especially crop disease working groups and by monitoring trade journals or literature including disease first reports from APS or new disease reports from the British Society for Plant Pathology (BSPP). Examples of emerging diseases of phytosanitary concern or potential phytosanitary concern in United States agriculture include: tomato brown rugose fruit virus (ToBRFV), cucumber green mottle mosaic virus (CGMMV), maize bacterial leaf streak, Goss’s wilt of maize, and tarspot of maize.
4. Meeting Phytosanitary requirements through laboratory testing, Field Inspections, and Seed Treatments
In import permits, importing countries list the conditions that exporting countries must meet before seed can enter the importing country. These conditions may be satisfied in several ways including phytosanitary field inspections, seed health testing or seed treatments.
Phytosanitary field inspections
Phytosanitary field inspections are conducted by systematically walking through production fields or greenhouses of seed parent plants and scouting for specific diseases or pests. Phytosanitary field inspections are conducted by plant pathologists, trained plant health professionals, or regulatory personnel. These professionals should have with them the tools and reference materials described in the above section on field diagnosis. Inspections are conducted during a period of active plant growth and timing is critical. Scouting too early can lead to missed late season diseases, while scouting too late can lead to missed diseases due to masking of symptoms by plant senescence. For most diseases, a scouting time or ideal phenological stage can be identified ahead of the growing season. Some import permits may specify inspection timing or phenological stage. A good rule of thumb for most diseases is to scout immediately prior to flowering or during flowering and seed formation. Specific diseases may require scouting at other phenological stages, such as scouting for sunflower downy mildew during the seedling stage. The phenological stage of the seed parent plant should be recorded in the inspection report. During inspections, areas of the field where plant stress is likely to occur, such as low-lying areas, areas of poor drainage, borders, and shaded areas, should be carefully observed. Seed increase plots are often small, and each individual plot should be inspected for adequate record keeping. For larger fields or plots, inspection patterns, such as W- or X-shaped, should be established. Record keeping is essential for successful field inspections. The seed lots produced from the inspected seed parent plants can be distributed for many years, so long-term management of inspection records is critical. Use of databases designed for germplasm management, such as GRIN-Global, can greatly help in record keeping for field inspection and seed testing data. More information on phytosanitary field inspections can be found on the National Seed Health System (NSHS) website.
Seed health testing
Seed health testing is essential to ensure that seeds moving into and out of germplasm collections carry a very low risk of pathogen transmission.
Seed health testing methods can be divided into direct testing methods or indirect testing methods. Direct testing methods confirm both the presence (is the pathogen there?), viability (is the pathogen alive?), and pathogenicity (can the pathogen cause disease?) of pathogens detected in or on seeds. Indirect testing methods confirm the presence of the pathogen but provide no information on pathogen viability or pathogenicity. Negative test results for both direct and indirect tests give good confidence that seeds are free from pathogens. Positive results of indirect tests usually require follow-up testing to prove that the detected pathogen is viable and pathogenic.
Seed health testing methods can be classified as serological (ELISA), molecular (PCR, qPCR, and high throughput sequencing), blotter, culture, grow-out, visual or microscopy. A brief description of selected methods is provided below and a comparison of the methods is provided in Table 2.
- Enzyme-linked immunosorbent assay (ELISA) is a serological method that uses pathogen-specific antibodies and an enzyme-linked antibody that can produce a color change when the enzyme’s substrate is added to the reaction.
- Polymerase chain reaction (PCR) is a molecular method of pathogen detection in which pathogen-specific DNA is amplified and later visualized using gel electrophoresis. Reverse transcriptase PCR (RT-PCR) is used for detection of RNA viruses.
- Quantitative PCR (qPCR) is a molecular method of pathogen detection in which pathogen-specific DNA is amplified and detected through use of fluorescent probes or fluorescent DNA-binding dyes.
- High throughput sequencing is a method of detecting pathogens that relies on advanced sequencing techniques to sequence all DNA present in a sample (metagenomic approach) or DNA of a specific group of microbes, such as bacteria or fungi (targeted amplicon sequencing). Analysis of high throughput sequencing datasets uses bioinformatics approaches to identify pathogens.
- In blotter-based methods of seed testing, seeds are placed in containers with moistened blotter paper and incubated under controlled temperatures for a period. Seeds are then observed for the presence of pathogens. Blotter-based methods can rely on freezing or herbicides to prevent seed germination.
- In culture-based methods of seed testing, seeds, seed extracts, or seed washes, are plated onto agar media for pathogen growth. Media can support the growth of a wide range of microbes (general media) or specific groups of microbes (semi-selective) media. Pathogens can have distinctive appearances on media (Figure 4).
- In grow-outs, a subsample of a seedlot is used to grow plants under conditions conducive to the development of disease symptoms. If disease symptoms develop in the plants, the pathogen most likely originated from the seedlot.
- In visual methods, seeds are observed for the presence of pathogen structures or damage.
- Microscopy-based methods are used to examine seeds or seed washes for the presence of pathogens under either dissecting or compound microscopes.
One difficulty of seed health testing in germplasm collections is selecting a seed testing sample size that will preserve valuable germplasm, while giving reasonable confidence in the testing results. For example, a 2018 protocol for testing Cucurbit seeds for Acidovorax citrulli, the causal agent of bacterial fruit blotch, recommends a sample size of 10,000-30,000 seeds for grow-out assays and 30,000 seeds for a qPCR-based approach. These sample sizes are likely larger than the size of an entire seed lot for some genebank accessions. Some importing NPPOs are strict with seedlot sample size requirements, but a seedbank plant pathologist should attempt to work with these NPPOs to gain adjustments or waivers to allow for germplasm importation.
Published seed health tests are available from the NSHS, International Seed Testing Association (ISTA), and International Seed Health Initiative for Vegetable-Crops (ISHI-Veg). Guidelines for best practices for seed health testing are also available from ISHI-Veg. ISTA maintains a list of seedborne diseases with relevant literature.
Table 2. A summary of the types of seed health testing methods and their associated characteristics. Expertise is ranked from low to high referring to the amount of training, skill and knowledge needed to conduct the method. Cost refers to the cost of running the method based on consumable supplies and does not refer to baseline equipment costs. Equipment refers to the amount of laboratory equipment required to conduct the test and how readily accessible it is in the lab. The final column describes whether or not the test can be used to assess pathogen viability.
| Test | Expertise required | Cost | Time | Equipment required | Proves pathogen viability? |
| ELISA | Low to moderate | Low to moderate | Hours to days | Low to Moderate | No |
| PCR/qPCR | Moderate to high | Moderate to high | Hours to days | High | No* |
| High throughput sequencing | High | High | Days to months | High | No |
| Blotter- or culture-based methods | Moderate to high | Low | Days to weeks | Low | Yes |
| Grow outs | Low to moderate | Moderate to high | Weeks to months | High** | Yes |
| Visual | Moderate to high | Low | Hours to days | Low | No |
| Microscopy | High | Low | Hours to days | Moderate | No*** |
*Use of propidium monoazide has been applied to differentiate between DNA in living versus dead cells in a limited number of PCR assays.
**Grow-outs can require a significant amount of space in controlled environments either in greenhouses, grow rooms or growth chambers.
***Although one cannot typically tell if pathogens are viable under the microscope, you can determine the viability of some pathogens, such as nematodes.
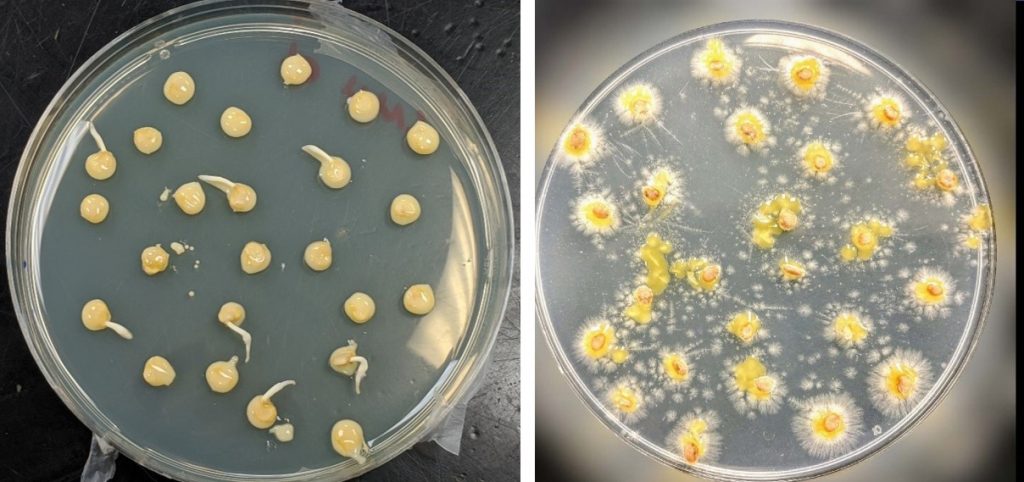
Figure 4. Examples of differences in appearance of bacteria on semi-selective media for pepper seed artificially infested with Clavibacter michiganensis subsp. michiganensis on D2ANX medium (left panel) and tomato seeds artificially infested with Xanthomonas perforans on CKTM medium (right panel). Photo credit: Dr. Francesca Rotondo, The Ohio State University.
Seed treatments
Seed treatments to eliminate or reduce pathogens on seed can consist of chemical or physical methods. For chemical methods, seeds can be exposed to liquids (such as a sodium hypochlorite (bleach) solution) or gasses (such as chlorine gas). Chemical seed treatments can also include application of fungicides. Fungicide seed treatments usually consist of a broad-spectrum fungicide, such as thiram. For oomycete diseases such as downy mildews, a more pathogen-specific fungicide, such as metalaxyl/mefenoxam is applied. Physical seed treatments usually involve the application of heat, either as a dry application in an oven, steam, or by submerging seeds in hot water. Heat treatments can be used to kill and inactivate fungi, oomycetes, bacteria, and viruses. While seed treatments can be used to meet phytosanitary requirements, they are also an important management strategy for reducing diseases in seed increases conducted in germplasm collections. Seed treatments should be applied as close to planting as possible, as many seed treatments can impact vigor and long-term seed viability. If seed treatments will be incorporated into seed processing prior to long-term storage, the effects of these treatments on long-term viability should be investigated prior to adoption of the seed treatment.
5. Research to Improve Health in Germplasm Collections
Plant pathologists also support germplasm collections through breeding and research to improve plant health. Plant pathologists may work directly to breed disease resistant varieties or support breeders through germplasm screening for disease resistance. Plant pathologists can also develop molecular markers of disease resistance loci to speed germplasm screening. Since seed health testing plays such an important role in germplasm distribution, developing new or improved seed health testing or disease diagnostics methods is a key part of research. Similarly, developing methods to reduce infection or infestation of plant pathogens on seeds is important to support healthy seed parent plant production within germplasm collections. Another avenue of research is development of disease management strategies for key diseases that affect taxa in the germplasm collection. Plant pathologists working in germplasm collections devoted solely to germplasm maintenance usually do not engage directly in more esoteric, academic fields of plant pathology, such as molecular plant microbe interactions. However, these plant pathologists can play important roles by sharing their knowledge of germplasm diversity while supporting and collaborating with researchers at institutions engaged in other avenues of research.
6. References
Agarwal, V.K. and Sinclar, J.B. 1996. Principles of Seed Pathology. CRC Press, Boca Raton, FL, USA.
Compendia of Plant Diseases and Pests. APS Press, St. Paul, MN, USA.
Fry, W.E. 1982. Principles of Plant Disease Management. Academic Press, New York City, USA.
Howard, R.J., Garland, J.A., and Seaman, W.L. editors. 1994. Diseases and Pests of Vegetable Crops in Canada. The Canadian Phytopathological Society and the Entomological Society of Canada, Ottawa, CA.
Neergard, P. 1977. Seed Pathology. Palgrave, London, UK.
Shurtleff, M.C. and Averre, C.W. 1997. The Plant Disease Clinic and Field Diagnosis of Abiotic Diseases. APS Press, St. Paul, MN, USA.
Streets, R.B. 1972. The Diagnosis of Plant Diseases. The University of Arizona Press, Tucson, AZ, USA.
Walters, D. 2009. Disease Control in Crops: Biological and Environmentally-Friendly Approaches. Wiley-Blackwell, West Sussex, UK.
7. Additional information
Acronym List
APS: American Phytopathological Society
BSPP: British Society for Plant Pathology
DNA: Deoxyribonucleic acid
CGMMV: Cucumber green mottle mosaic virus
ELISA: Enzyme linked immunosorbent assay
GRIN: Germplasm Resources Information Network
HTS: High throughput sequencing
IDALS: Iowa Department of Agriculture and Land Stewardship
IPPC: International Plant Protection Convention
ISHI-Veg: International Seed Health Initiative for Vegetable Crops
ISPM: International Standards for Phytosanitary Measures
ISTA: International Seed Testing Association
NAPPO: North American Plant Protection Organization
NCRPIS: North Central Regional Plant Introduction Station
NSHS: National Seed Health System
NPPO: National Plant Protection Organization
PCR: Polymerase chain reaction
PPO: Plant Protection Organization
QPCR: Quantitative Polymerase Chain Reaction
RNA: Ribonucleic acid
RPPO: Regional Plant Protection Organization
RT-PCR: Reverse transcriptase polymerase chain reaction
ToBRFV: Tomato brown rugose fruit virus
USDA-APHIS: United States Department of Agriculture Animal and Plant Health Inspection Service
USDA-ARS: United States Department of Agriculture Agricultural Research Service
USDA-NRCS: United States Department of Agriculture Natural Resources Conservation Service
8. Acknowledgments
Citation: Testen A. 2021. Maintaining and ensuring plant health in seed germplasm collections: an applied plant pathology perspective. In: Volk GM (Ed.) Addressing pathology issues in plant genebanks. Date accessed. Available from https://colostate.pressbooks.pub/plantgenebankpathology/chapter/maintainingandensuringplanthealth/
This training module was made possible by:
Editors: Gayle Volk, Katheryn Chen
Content provider: Anna Testen
Reviewers: Charles Block, Peter Bretting, Tracy Bruns, Lisa Burke, Candice Gardner, Robert Krueger, Joseph Postman, and Ashley Sonner
This project was funded by the USDA-ARS and grant 2020-70003-30930 from the USDA-NIFA-Higher Education Challenge Grant Program. USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.

