Prunus Shoot Tip Cryopreservation

Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation (retired), 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@colostate.edu
Remi Bonnart, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521.
Outline
- Introduction
- Establishment of in vitro cultures
- Shoot tip excision
- Shoot tip processing and liquid nitrogen exposure
- Long-term storage
- Viability assessment
- References
- Additional information
- Acknowledgments
The Prunus cryopreservation procedure is available for download here.
1. Introduction
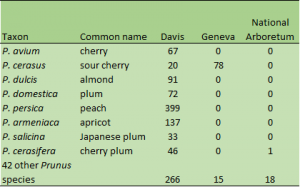
The USDA-ARS National Plant Germplasm System has a field collections of Prunus trees (Fig. 1) at the National Clonal Germplasm Repository for Tree Fruits and Nut Crops and Grapes in Davis, California (Fig. 2) and a sour cherry orchard collection at the Plant Genetic Resources Unit in Geneva, New York, as well as a collection of Prunus ornamentals at the National Arboretum in Washington, D.C. These collections are all field-maintained and only a few accessions in the collections are currently backed-up in the base collection at the National Laboratory for Genetic Resources Preservation (NLGRP) in Fort Collins, Colorado. A method for cryopreserving shoot tips of Prunus trees (sweet cherry and peaches) was developed at the NLGRP to preserve Prunus cultivars in liquid nitrogen.
Figure 1. Number of Prunus accessions maintained at the Davis (CA), Geneva (NY), and National Arboretum (Washington, D.C.) locations in the National Plant Germplasm System.
Figure 2. Prunus trees in the NPGS field collection in Davis, California. Photo credit: Gayle Volk.
2. Establishment of in vitro cultures
In vitro cultures are established from field-grown trees. Young flushes of growth (Fig. 3) are harvested after budbreak in the springtime. The leaves are removed from the young branch and the branch is cut into pieces for surface sterilization. Tissue sections are surface sterilized with 70% isopropanol for 3 minutes and then rinsed three times with tap water. The nodal sections are then treated with 10% bleach (0.825% sodium hypochlorite final concentration) for 10 minutes and transferred into a laminar flow hood. They are then rinsed three times with sterile, distilled water. Each nodal section is placed into a test tube containing Prunus Shoot Maintenance Medium (Murashige and Skoog (MS) salts, 30 g L-1 sucrose, 0.25 mg L-1 6-benzylaminopurine, 0.5 mg L-1 kinetin, 7 g L-1 agar, pH 5.7). The process of surface sterilization is shown in Video 1. Non-contaminated cultures are multiplied until there is enough plant material to harvest shoot tips for cryopreservation. In vitro cultures (Fig. 4) are grown at 25 oC with an 18 hour photoperiod with 85 µmol m-2 s-1 light provided by fluorescent bulbs.
Figure 3. Branches of Prunus trees with young growth appropriate for introduction into tissue culture. Photo credit: Remi Bonnart.
Video 1. Surface sterilization of Prunus shoots for introduction into tissue culture, demonstrated by Remi Bonnart.
Figure 4. Prunus persica (peach, left) and Prunus avium (sweet cherry, right) cultures as sources of shoot tips for cryopreservation. Photo credit: Gayle Volk.
3. Shoot tip excision
Two millimeter shoot tips are excised from the apical buds derived from 4- to 6- week old in vitro-grown plants. The apical shoot tips are plated onto Prunus Preculture Medium (1/2 strength MS, 0.3 M sucrose, 0.4 M proline, 0.1 mM salicylic acid, 1 mM glutathione (reduced form), 8 g L-1 agar, pH 5.7) for three days at 25 oC in the dark. Shoot tip excision is demonstrated in Video 2.
Video 2. Excision of Prunus persica shoot tips, demonstrated and narrated by Remi Bonnart (NLGRP).
4. Shoot tip processing and liquid nitrogen exposure
Shoot tips are removed from the Preculture Medium and placed in Loading Solution (2 M glycerol + 0.4 M sucrose + 1/2 strength MS salts) for 20 minutes at 22 oC. The Loading Solution is then removed and replaced with Plant Vitrification Solution 2 (PVS2; Sakai et al. 1990) at 0 oC for 75 to 90 minutes. The shoot tips are then placed onto a thin later of PVS2 on foil strips, plunged into liquid nitrogen (LN), and transferred to labeled cryovials (Video 3).
Video 3. Video of the transfer of shoot tips from Prunus Preculture Medium into Loading Solution, then cryopreserve using a droplet vitrification technique, demonstrated and narrated by Remi Bonnart (NLGRP).
5. Long-term storage
Cryovials (Fig. 5) are placed onto cryocanes (Fig. 6), which are then placed into labeled sleeves in cans. They will be maintained in the liquid or vapor phase of liquid nitrogen for long-term storage (Fig. 7). This process is shown in Video 4. A total of 170 shoot tips are processed per accession, with 10 shoot tips placed on each foil, and one foil placed into each cryovial. Fifteen cryovials (of 10 shoot tips each) are cryopreserved in the base collection, and one cryovial is warmed for a recovery event. The remaining 2 cryovials are available for viability assessments.
Figure 5. Cryovial for Prunus shoot tip cryopreservation. Photo credit: Remi Bonnart.
Figure 6. Cryovials are loaded onto cryocanes for long-term storage. Photo credit: Gayle Volk.
Figure 7. Aluminum cryoboxes in the vapor phase of liquid nitrogen. Photo credit: Gayle Volk.
Video 4. Cryovials with shoot tips are transferred from the dewar to the liquid nitrogen tanks for long-term storage, demonstrated and narrated by Remi Bonnart (NLGRP).
6. Viability assessment
Shoot tips are warmed by removing the foil strips from the cryovials and then placing the foil strips into room temperature Unloading Solution (1/2 strength MS + 1.2 M sucrose, pH 5.7) for 20 minutes. Shoot tips are then plated onto Prunus Recovery Medium #1 (1/2 strength MS macro elements (-NH4), MS microelements and vitamins, 30 g L-1 sucrose, 0.1 mg L-1 6-benzylaminopurine, 0.1 mg L-1 kinetin, 0.1 mg L-1 GA3, 8 g L-1 agar, pH 5.7). This process is shown in Video 5. After two weeks of culture in the dark on Prunus Recovery Medium #1, shoot tips are transferred to Prunus Recovery Medium #2 (1/2 MS macro elements (+NH4) + MS micro elements and vitamins, 30 g L-1 sucrose, 0.25 mg L-1 6-benzylaminopurine, 0.25 mg L-1 kinetin, 0.1 mg L-1 GA3, 8 g L-1 agar, pH 5.7). Shoot tips are then grown at 50 µmol m-2 s-1 provided by fluorescent lights. Shoot tips are grown for 8 weeks and transferred to fresh medium as needed (P. persica was transferred biweekly). Shoot tip survival and regrowth data are collected after 8 weeks (Video 6 and 7).
Video 5. Warming and plating cryopreserved Prunus shoot tips, demonstrated and narrated by Remi Bonnart (NLGRP).
Video 6. Recovery of Prunus persica shoot tips exposed to liquid nitrogen. Images were taken weekly for 6 weeks.
Video 7. Recovery of Prunus persica shoot tips, non-cryopreserved controls. Images were taken weekly for 6 weeks.
7. References
Sakai A, Kobayashi S, Oiyama I. 1990. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. Var. Brasiliensis Tanaka) by vitrification. Plant Cell Rep. 9(1):30-33. doi: 10.1007/BF00232130
8. Additional information
The Prunus cryopreservation procedure is available for download here.
9. Acknowledgments
Citation: Volk GM, Bonnart R. 2020. Prunus Shoot Tip Cryopreservation. In: Volk GM (Eds.) Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules. Fort Collins, Colorado: Colorado State University. Date accessed. Available from https://colostate.pressbooks.pub/clonalcryopreservation/chapter/prunus-cryopreservation/
This training module was made possible by:
Editors: Emma Balunek, Gayle Volk
Content providers: Remi Bonnart, Gayle Volk
Videographers: Mike May, Remi Bonnart, Ashley Shepherd, Gayle Volk
This project was funded in part by the National Academy of Sciences (NAS) and USAID, and any opinions, findings, conclusions, or recommendations expressed in such are those of the authors alone, and do not necessarily reflect the views of USAID or NAS. USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.