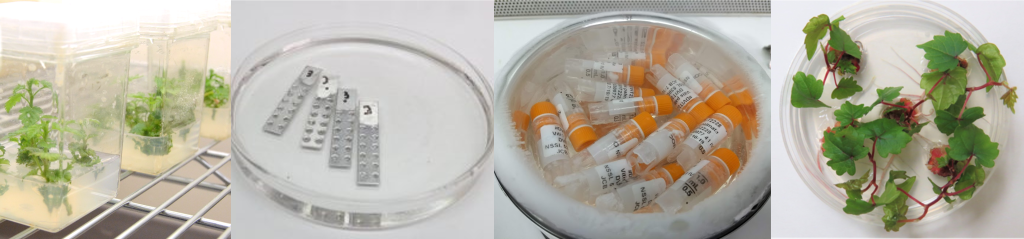
An Overview of Shoot Tip Cryopreservation
Jean Carlos Bettoni, Independent Researcher, 35 Brasil Correia Street, Videira, SC 89560510, Brazil. jcbettoni@gmail.com
Katheryn Chen, Department of Soil and Crop Sciences, Colorado State University, 307 University Ave., Fort Collins, Colorado 80523.
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
The purpose of this chapter is to present an overview of the steps involved in plant shoot tip cryopreservation and to highlight some of the fundamental concepts behind these procedures.
Outline
- Introduction
- Plant material
- Shoot tip excision
- Cryopreservation process
- Recovery process
- References
- Acknowledgements
1. Introduction
Shoot tips are organized tissues composed of an apical dome (made of meristematic cells with dense cytoplasm and small vacuoles) and several leaf primordia (Figure 1). These tissues can regenerate entire plants with a high level of genetic fidelity. Shoot tip cryopreservation procedures have been established for a wide variety of vegetatively propagated plants and are often used by genebanks to conserve clonal cultivars and other essential plant genetic resources.
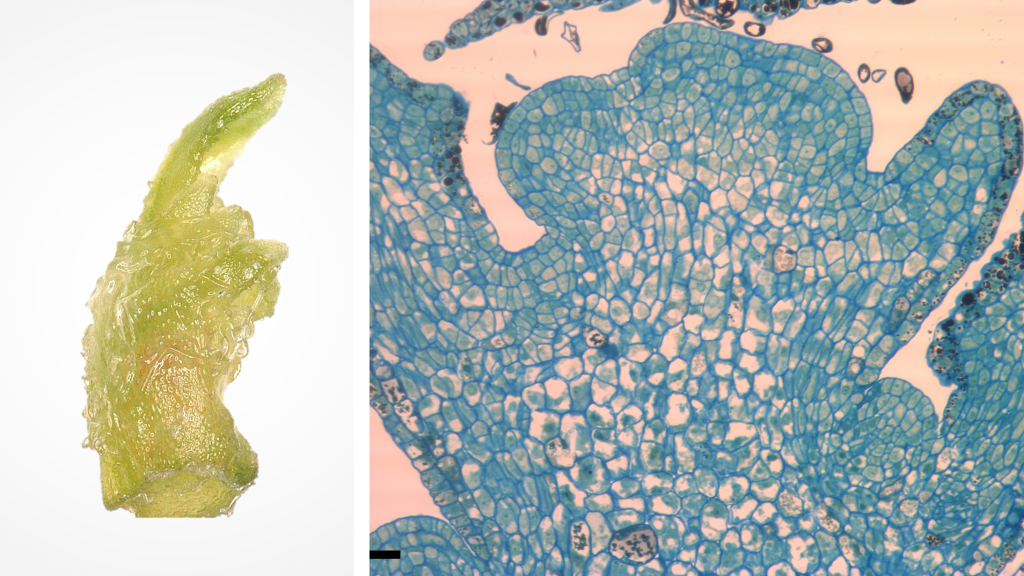 Figure 1. Grape shoot tip (1 mm, left) and cross section (right). Photo credit: J.C. Bettoni.
Figure 1. Grape shoot tip (1 mm, left) and cross section (right). Photo credit: J.C. Bettoni.
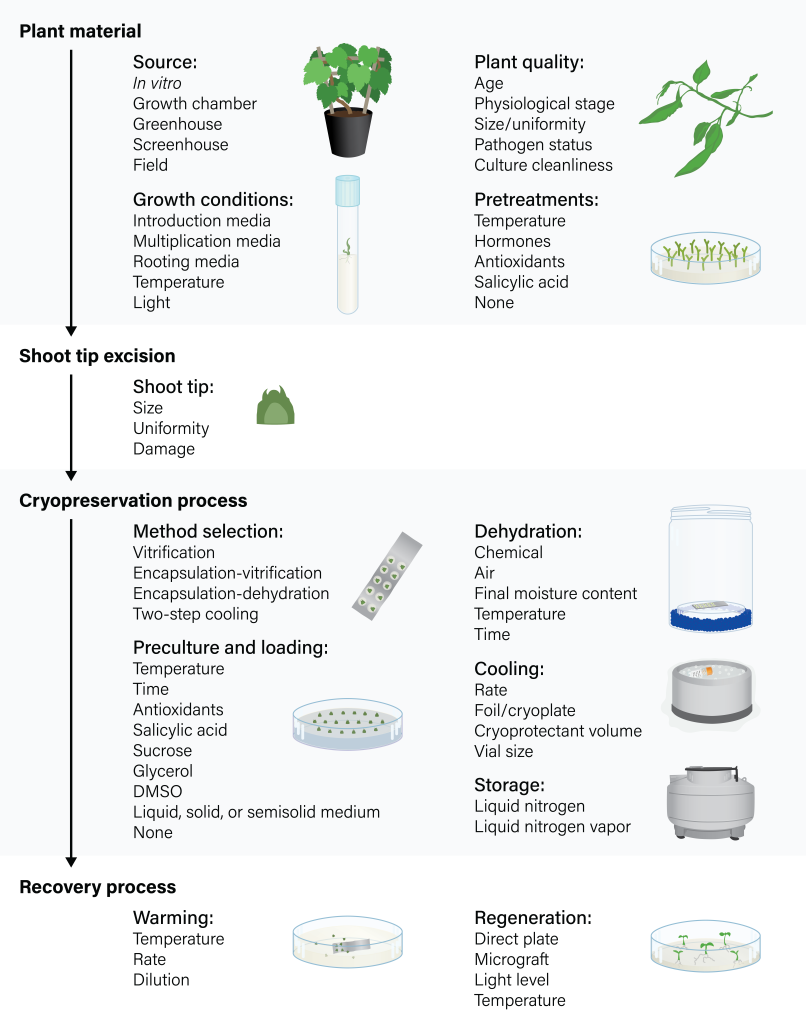
Cryopreserving shoot tips is a complex, multi-step process. Because no single procedure can be easily implemented across a wide range of plant species, cryopreserving new material means that each step must be optimized for it to retain its capacity to generate healthy plants after cryoexposure. Protocols might specify factors related to donor cultures, micropropagation systems, shoot tip excision, pretreatments and preculture conditions, cryopreservation method, and recovery process (Figure 2). It is therefore essential to understand the function of each step and how it will impact the outcome of cryopreservation.
Figure 2. Flowchart describing the critical steps and related factors for shoot tip cryopreservation. Figure by K. Chen, adapted from Bettoni et al., 2021.
2. Plant material
2.1. Source, plant quality, and growth conditions
Successful shoot tip cryopreservation relies on high quality source plants. Shoot tips can be excised from surface‐sterilized plants grown in the field, greenhouse/screenhouse, growth chamber, or most commonly from in vitro cultures (Figure 3). Source plants should preferably be free of pathogenic and endophytic fungi, bacteria, viruses and viroids. In some cases, additional treatments might be required to remove endophytes (Volk et al., 2022). Many other factors related to the donor cultures—such as age, physiological stage, light conditions, and overall health—influence the recovery of cryopreserved shoot tips (Zhang et al., 2023).
 Figure 3. Shoot tips can be excised from plants grown in the field (such as garlic, left), greenhouse or screenhouse (such as citrus, middle), or in vitro (such as apple, right). Photo credit: G. Volk.
Figure 3. Shoot tips can be excised from plants grown in the field (such as garlic, left), greenhouse or screenhouse (such as citrus, middle), or in vitro (such as apple, right). Photo credit: G. Volk.
When tissue cultures serve as source material, it is important to use those that have been introduced into culture relatively recently to reduce the opportunities for somaclonal variation. Tissue culture plants are multiplied to produce a sufficient quantity of shoot tips for cryopreservation. These can either be excised directly, or undergo any number of intermediate steps, known as pretreatments, in preparation for cryopreservation.
Depending on the cleanliness of source material as well as a number of other factors, some protocols opt to eliminate the initial step of tissue culture introduction. By excising shoot tips directly from surface sterilized plants, processing time and resources can be greatly reduced. Regardless of explant source, all shoot tip cryopreservation protocols incorporate tissue culture to some extent; therefore, the development of successful micropropagation systems is critical for cryopreserving shoot tips (Bettoni et al., 2021).
2.2 Pretreatments
Source plants might need pretreatments for successful cryopreservation. These are procedures performed on the original source materials, either in vitro or in vivo, prior to shoot tip excision. Pretreatment protocols must be optimized for each plant species, as they might require differing growth conditions. Plants can be subjected to one or several of these treatments, applied successively or concomitantly.
Pretreating source plants through cold acclimation and/or exposure to high sugar levels enhances the physiological tolerance to the dehydration process. Cold hardening of the source plants has been routinely applied for some woody species; its effectiveness depends on optimizing the duration of chilling (often at 5 °C), which can be genotype-dependent. Alternatively, source plants can be grown on media supplemented with osmotic agents, salicylic acid, hormones, and antioxidants to induce the optimal physiological state for successful dehydration and cryopreservation of some crops (Wang et al., 2018; Bettoni et al., 2021).
Another possible step is to plate nodal sections onto a medium prior to apical shoot tip excision (Figure 4). This is an effective way to achieve high-quality, uniform shoot tips. This simple step minimizes the physiological effects of apical dominance in in vitro stock cultures and ensures the production of numerous apical shoot tips (Bettoni et al., 2019). Nodal sections can be sourced directly from greenhouse plants (Figure 5B), but are more commonly derived from tissue cultures (Figure 5C).
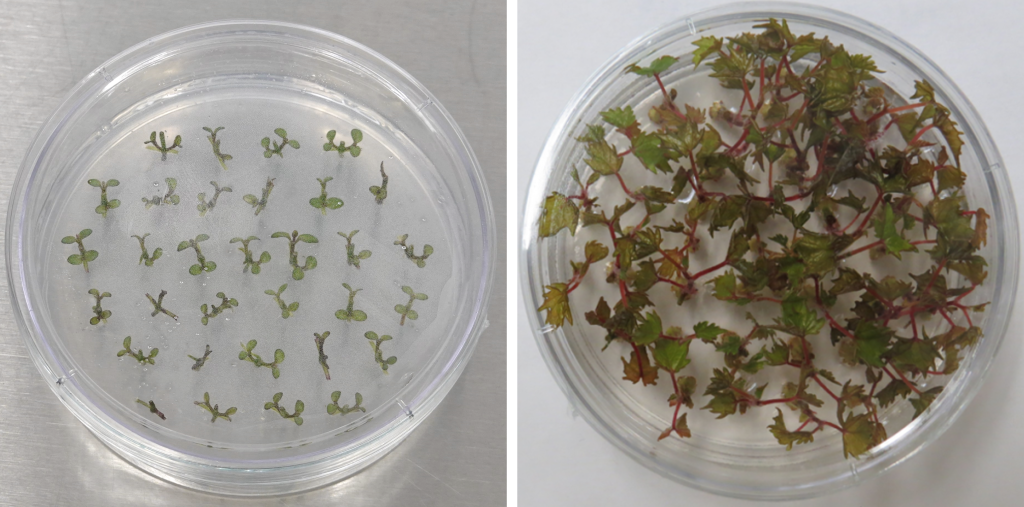 Figure 4. Nodal sections of mint (left) and grape (right) prior to shoot tip excision. Photo credit: K. Chen (left) and J.C. Bettoni (right).
Figure 4. Nodal sections of mint (left) and grape (right) prior to shoot tip excision. Photo credit: K. Chen (left) and J.C. Bettoni (right).
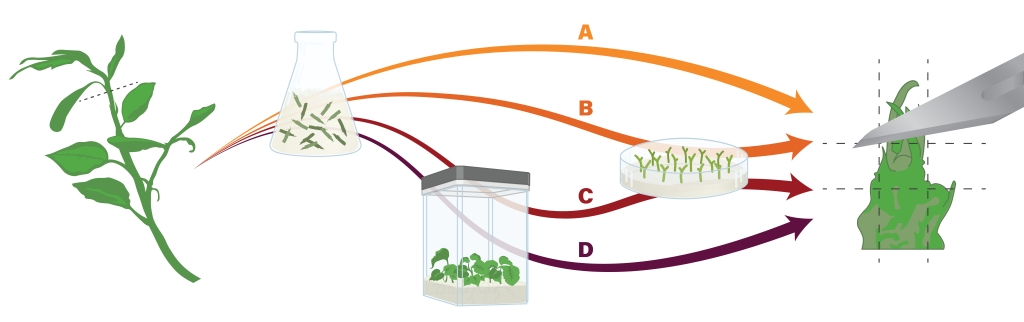 Figure 5. In cryopreservation procedures, production and preparation of shoot tips begins with tissues sourced from field or greenhouse-grown plants . These are surface sterilized, and then (A) shoot tips are excised directly, (B) plated and grown as nodal sections before shoot tips are excised, (C) initiated into tissue culture, followed by nodal sections, followed by shoot tip excision, or (D) initiated into tissue cultures that are used directly for apical shoot tip excision. Figure by K. Chen.
Figure 5. In cryopreservation procedures, production and preparation of shoot tips begins with tissues sourced from field or greenhouse-grown plants . These are surface sterilized, and then (A) shoot tips are excised directly, (B) plated and grown as nodal sections before shoot tips are excised, (C) initiated into tissue culture, followed by nodal sections, followed by shoot tip excision, or (D) initiated into tissue cultures that are used directly for apical shoot tip excision. Figure by K. Chen.
3. Shoot tip excision
After any pretreatments, small, uniform shoot tips are carefully excised. The size of excised shoot tips affects the optimal cryoprotection conditions. Depending on the species, 1–3 mm shoot tips are most commonly targeted (Bettoni et al., 2021). Small explant sizes facilitate the cryoprotectant uptake, consequently improving water removal. However, tiny shoot tips require extra care to reduce mechanical injury from handling, and loss of shoot tips during the course of the cryopreservation protocol. Larger shoot tips might not dehydrate evenly and could die from ice crystal formation, and the adjacent cells can undergo programmed cell death, resulting in poor regrowth.
Shoot tip excision is a practiced skill. In addition to producing accurate and uniform shoot tips, very precise cuts are needed to reduce mechanical damage and improve post-cryopreservation regrowth. Excisions must also be performed quickly to reduce the risk of tissue dehydration; this can be a difficult undertaking when large volumes of material are processed.
4. Cryopreservation process
Once excised, shoot tips can undergo dramatically different treatments depending on the selected preservation protocol. In all cases, these series of treatments are designed to prepare shoot tips for the extreme temperature change that occurs with liquid nitrogen exposure. Without treatment, the cooling process can cause irreversible damage; intracellular water can freeze, forming lethal ice crystals that rupture cell membranes and cause leakage of intracellular components. Many shoot cryopreservation protocols reduce and/or remove water content from the cells through either physical or osmotic dehydration, which leads to vitrification of the highly concentrated cytoplasm. Encapsulation-dehydration methods achieve this by partially drying tolerant tissues, while vitrification methods incorporate permeable/colligative cryoprotectant solutions that osmotically remove most water from cells and change the freezing properties of the remaining water. The accurate control of dehydration and the prevention of excessive osmotic stress are crucial for successful shoot tip cryopreservation (Volk and Walters, 2003).
4.1. An overview of methods
Two-step cooling
Shoot tip cryopreservation procedures initially followed two-step cooling protocols developed for cell suspensions. In these methods, shoot tips are treated with low concentrations of cryoprotectants (often including polyethylene glycol, glucose, and dimethyl sulfoxide), slowly cooled to a critical temperature, and then plunged into liquid nitrogen (LN). This approach relies on freeze-induced dehydration as water is drawn out of the shoot tips over the several hours of slow cooling. Slow cooling typically occurs in a programmable-controlled freezer at rate of 0.1 to 1 °C min-1, until they reach -30 °C (Matsumoto and Niino, 2014; Volk and Walters, 2003).
Two-step cooling can be an effective method for retaining the integrity of individual cells; however, in the case of differentiated tissues such as shoot tips, it is less efficient in retaining the tissue integrity needed for meristem survival and can result in shoot growth with intermediate callus formation. The field of plant cryopreservation has achieved progress through adapting classical protocols such as two-step cooling, thereby creating simplified, high-throughput methods that enable the preservation of more plant species. Currently, most shoot tip cryopreservation procedures for vegetatively propagated crops follow protocols based on encapsulation-dehydration and vitrification.
Encapsulation-dehydration
The encapsulation-dehydration technique is based on the technology developed for producing artificial seeds. Shoot tips are embedded into calcium alginate beads and treated with sucrose-enriched medium at either fixed or increasing concentrations for several hours or days. The beads are then physically dehydrated by either air-drying in the air current of a laminar flow hood (Figure 6) or with silica gel. The beads are loaded into vials, followed by rapid freezing in LN (Matsumoto and Niino, 2014). Although encapsulation-dehydration is relatively straightforward, it is a labor-intensive and time-consuming technique, and is only successful for species that tolerate high sucrose concentrations.
 Figure 6. Shoot tips encapsulated in calcium alginate beads, air drying in laminar flow hood. Technique performed by Remi Bonnart. Photo credit: K. Chen.
Figure 6. Shoot tips encapsulated in calcium alginate beads, air drying in laminar flow hood. Technique performed by Remi Bonnart. Photo credit: K. Chen.
The recently developed Dehydration cryoplate (D cryoplate) protocol, modifies traditional encapsulation-dehydration by adding aluminum cryoplates; shoot tips are enclosed in tiny drops of calcium alginate and placed on the aluminum plate before being air-drying dehydrated, and subsequently exposed directly to LN (Niino et al., 2019).
Vitrification
Vitrification methods are currently the most widely applied approach to cryopreserving plant shoot tips. Successful vitrification requires osmotic dehydration of cells prior to direct exposure to LN; this is achieved by exposing shoot tips to high concentrations of cryoprotectants to increase the viscosity of the cell solution and promote the formation of a glassy state (Volk et al., 2006). Because many plant species cannot be successfully cryopreserved if directly exposed to highly concentrated cryoprotectants, protocols typically require additional pretreatment and preculture steps. To enhance tolerance to the dehydration process, donor plants can be exposed to cold or osmotic acclimation before shoot tip excision, and shoot tips are often precultured on a medium with high sucrose concentration and subsequently transferred to a glycerol-sucrose solution (Bettoni et al., 2021).
Vitrification methods traditionally place shoot tips directly into the cryovial along with a cryoprotectant; however, many successful variations have been developed including encapsulation-vitrification, droplet-vitrification, vitrification cryoplates (V cryoplate), and other variations (Figure 7). Comprehensive information about vitrification and encapsulation-based cryopreservation methods can be found in several reviews (Matsumoto, 2017; Wang et al., 2018; Niino et al., 2019; Bettoni et al., 2021; Zhang et al., 2023).
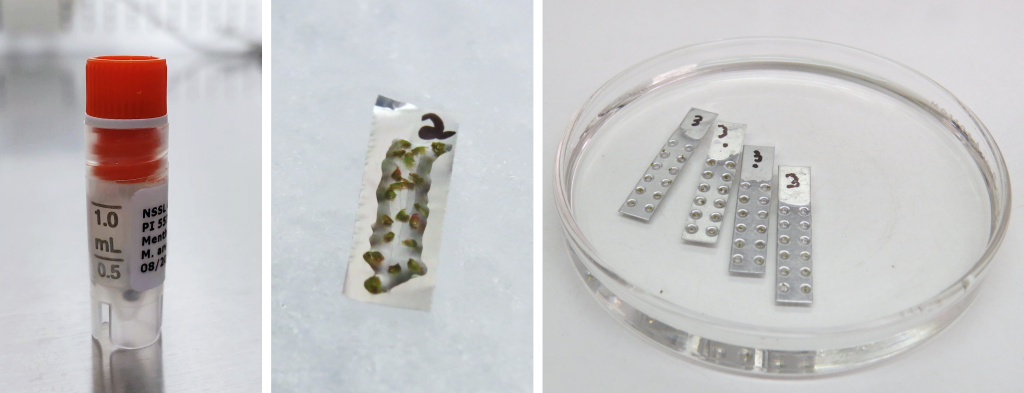 Figure 7. Vitrification in a vial (left), droplet-vitrification on a foil strip (middle), and vitrification cryoplates (right). Photo credit: K. Chen (left), J.C. Bettoni (middle, right).
Figure 7. Vitrification in a vial (left), droplet-vitrification on a foil strip (middle), and vitrification cryoplates (right). Photo credit: K. Chen (left), J.C. Bettoni (middle, right).
4.2. Preculture and loading solutions
Preculture conditions play a major role in improving shoot tip survival and regrowth capacity after cryopreservation. In most cases, exposure to a cryoprotectant without this step can cause desiccation damage to cell membranes due to a high concentration of internal solutes and protein denaturation. Preculture solutions are designed to increase shoot tip tolerance to chemical or physical dehydration, as well as cooling stress.
Shoot tips are typically incubated in sucrose-enriched media (0.3 to 1.0 M sucrose) and/or sometimes other additives such as glycerol or dimethyl sulfoxide (DMSO) for hours to days prior to air-drying or exposure to cryoprotectants. This step induces the excised shoot tips to increase solute concentrations (sugar, starch, and proline), reduce water content, and enhance the stability of membrane and protein under conditions of severe dehydration (Zhang et al., 2023; Volk et al., 2018). Optimizing sucrose concentrations and the duration and conditions during preculture are important factors for enhancing tolerance to dehydration and subsequent freezing processes.
In vitrification solution-based methods, shoot tips are often precultured in a medium supplemented with 0.3 M sucrose prior to osmoprotection on loading solution, which usually applies sucrose and glycerol (often at 2 M glycerol and 0.4 M sucrose) to induce tolerance and mitigate the osmotic shock to the subsequent vitrification treatment (Matsumoto, 2017; Bettoni et al., 2019). Some successful cases incorporate a solution that combines the preculture and the loading treatments or without the need of osmoprotection (Li et al., 2015). Recently, the addition of antioxidants (such as ascorbic acid, glutathione, etc.) and elicitors of defense proteins (salicylic acid) to preculture media have significantly improved shoot tip regrowth after cryoexposure. This regrowth increase is correlated with a decrease in the level of oxidative stress to the cryopreserved shoot tips (Bettoni et al., 2021; Ren et al., 2021).
4.3. Dehydration techniques
Successful plant cryopreservation techniques rely on the removal of most if not all freezable water from shoot tip cells prior to the LN exposure. Inappropriate desiccation can adversely affect shoot tip regrowth after cryoexposure; therefore, suitable dehydration by either chemical or evaporative dehydration is one of the main steps to ensure success.
The success of the encapsulation-dehydration protocol depends on the extent of dehydration and the residual moisture content of the encapsulated beads before freezing. Shoot tips are encapsulated in calcium-alginate (2.0–3.0%, w/v) beads prior to physical dehydration by either air-drying in the air current of a laminar flow hood or with silica gel to an optimum moisture content (20–30%, fresh weight basis), which varies according to the species (Figure 6; Wang et al., 2018; Zhang et al., 2023).
Vitrification-based protocols make use of highly concentrated mixtures of cryoprotectants, also called vitrification solutions, that replace cellular water and promote glass formation within cells during the cooling process to avoid ice crystallization (Volk and Walters, 2006). Plant Vitrification Solution 2 (PVS2) is a commonly used cryoprotectant that has been highly effective for a wide range of plant genera. PVS2 contains 30% (w/v) glycerol, 15% (w/v) ethylene glycol (EG), 15% (w/v) DMSO, and 0.4 M sucrose in a liquid medium (Sakai et al., 1990). Penetration of some components of PVS2 (such as DMSO, EG, and glycerol) into the cytoplasm is key to its cryoprotective function (Video 1). Another frequently used cryoprotectant is Plant Vitrification Solution 3 (PVS3). PVS3 is composed of 50% (w/v) sucrose and 50% (w/v) glycerol and functions by creating an osmotic gradient that removes water from the cells (Nishizawa et al., 1993).
Video 1. Heidi Kreckel describes how plant cryoprotectant solutions interact with cells.
As is the case with each step in the preservation process, dehydration procedures require optimization for different plant species. Shoot tips are either directly immersed in full-strength PVS2, or the PVS2 concentration is increased stepwise. They may be incubated at room temperature or at 0 °C. Because PVS2 is known to be toxic to shoot tip cells, 0 °C exposure is often recommended to cope with the cytotoxicity (Bettoni et al., 2021). Incubation time in PVS2 must be precisely controlled according to numerous factors: the species, conditions and source of the explant, pretreatment and preculture conditions, shoot tip size, temperature, as well as the osmoprotection and cryopreservation protocol (Bettoni et al., 2021; Volk and Walters, 2006; Volk et al., 2006).
4.4. Cooling and storage
Cell survival is related to the rate at which explants are cooled to cryogenic temperatures. The type of container and amount of PVS significantly affect the cooling rate and, consequently, the levels of shoot tip regrowth after cryo-exposure. In traditional vitrification methods, several shoot tips are transferred to labeled cryovials containing 0.5–1.5 mL of fresh vitrification solution (Figure 8A). Encapsulation-vitrification methods embed shoot tips in beads, and similarly place them in cryovials filled with PVS. These methods rely on cryovials as the cooling vessel.
When shoot tips are instead contained within a small volume (1–5 μL per shoot tip) of the cryoprotective medium on aluminum foil (droplet-vitrification; Figure 8B), or attached to cryoplates that are submerged in PVS2 and then blotted to remove excess cryoprotectants (V cryoplate; Figure 8C) significantly faster cooling rates are achieved (Niino et al., 2019; Matsumoto, 2017). These strips/plates are plunged directly into LN, then subsequently loaded into cryovials. Furthermore, shoot tips cryopreserved on foils or cryoplates also experience faster warming rates during the recovery process. Achieving fast cooling and warming rates is one of the keys to the success of the droplet-vitrification and V cryoplate techniques. These efficient methods are highly valuable for cryopreserving plant germplasm within genebanks (Panis et al., 2020).
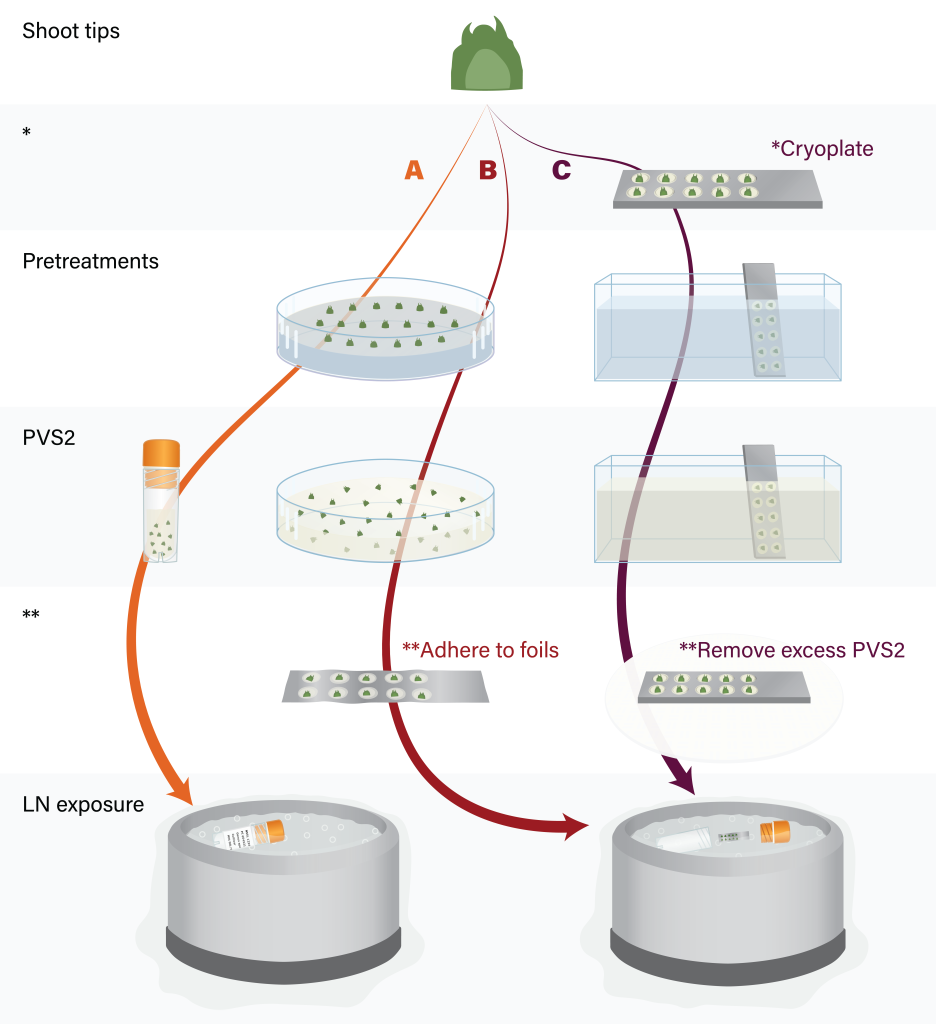 Figure 8. Major steps of shoot tip cryopreservation by vitrification (A), droplet-vitrification (B) and V cryoplate (C). Figure by K. Chen.
Figure 8. Major steps of shoot tip cryopreservation by vitrification (A), droplet-vitrification (B) and V cryoplate (C). Figure by K. Chen.
Once material has been exposed to LN, it is vital that it not experience rewarming until it is needed for viability assessments or regeneration. Vials must quickly be placed onto frozen cryocanes or into cryoboxes. Material should ideally remain in a temporary LN holding tank until the viability levels are determined. Once viability standards are met, those samples can be moved from the temporary tanks into long-term storage tanks.
5. Recovery process
5.1 Warming
All shoot tip cryopreservation methods require a successful recovery system. This process begins with warming, typically at a rapid rate. Shoot tips cryopreserved in cryovials can be warmed by placing at 38–40 °C for 2–3 min (encapsulation-dehydration), and washed in unloading solution with 0.3–1.2 M sucrose (vitrification and encapsulation-vitrification methods) or by immersing the foil strip or cryoplate containing shoot tips directly into unloading solution (droplet-vitrification and cryoplate methods) at room temperature or pre-warmed solution (35–40 °C).
5.2 REGENERATION
Favorable post-thaw conditions are essential for healthy shoot tip regrowth without intermediate callus formation. For many plants including mint, strawberry, and grape, this is achieved through direct recovery onto a medium; however, in specific cases such as citrus, they are micrografted onto compatible in vitro rootstocks (Popova et al., 2023; Bettoni et al., 2021). Significant improvements in regrowth have been achieved by culturing with gradual reductions in sucrose or the elimination of ammonium during the first few days after warming. It is also necessary to optimize the concentration and type of hormones included in the recovery medium. In many cases, shoot tips are maintained in the dark for the first two weeks before being transferred to normal light conditions (Volk et al., 2018; Bettoni et al., 2019, 2021; Zhang et al., 2023).
The ability to regenerate a plant in the greenhouse or field is at the core of plant cryopreservation; however, for the purpose of viability assessment, cultures generally do not need to be maintained to the point of acclimating and establishing them in the field. Because survival without shoot growth and elongation is not predictive that the material is able to be recovered into a complete plant, it is recommended that data be collected through shoot regrowth (Figure 9), usually several weeks depending on the species.
 Figure 9. Vitis plants recovered after droplet-vitrification cryopreservation. Photo credit: J.C. Bettoni.
Figure 9. Vitis plants recovered after droplet-vitrification cryopreservation. Photo credit: J.C. Bettoni.
6. References
Bettoni JC, Kretzschmar AA, Bonnart R, Shepherd A, Volk GM. 2019. Cryopreservation of 12 Vitis Species Using Apical Shoot Tips Derived from Plants Grown In Vitro. HortScience 54:976-981. DOI: 10.21273/HORTSCI13958-19
Bettoni JC, Bonnart R, Volk GM. 2021. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell, Tissue and Organ Culture 144:21-34. DOI: 10.1007/s11240-020-01846-x
Li BQ, Feng CH, Wang MR, Hu LY, Volk G, Wang QC. 2015. Recovery patters, histological observations and genetic integrity in Malus shoot tips cryopreserved using droplet-vitrification and encapsulation-dehydration procedures. Journal of Biotechnology 214:182-191. DOI: 10.1016/j.jbiotec.2015.09.030
Matsumoto T. 2017. Cryopreservation of plant genetic resources: conventional and new methods. Reviews in Agricultural Science 5:13-20. DOI: 10.7831/ras5.13
Matsumoto T, Niino T. 2014. The development of plant vitrification solution 2 and recent PVS2-based vitrification protocols. Acta Horticulturae 1039:21-28. DOI: 10.17660/ActaHortic.2014.1039.1
Niino T, Yamamoto S, Matsumoto T, Engelmann F, Valle Arizaga M, Tanaka D. 2019. Development of V and D cryo-plate methods as effective protocols for cryobanking. Acta Horticulturae 1234:249-262. DOI: 10.17660/ActaHortic.2019.1234.33
Nishizawa S, Sakai A, Amano Y, Matsuzawa T. 1993. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Science 91:67-73. DOI: 10.1016/0168-9452(93)90189-7
Panis B, Nagel M, Van den houwe I. 2020. Challenges and prospects for the conservation of crop genetic resources in field genebanks, in in vitro collections and/or in liquid nitrogen. Plants 9:1634. DOI: 10.3390/plants9121634
Popova E, Kulichenko I, Kim H-H. 2023. Critical role of regrowth conditions in post-cryopreservation of in vitro plant germplasm. Biology 12, 542. DOI: 10.3390/biology12040542
Ren L, Wang M-R, Wang Q-C. 2021. ROS-induced oxidative stress in plant cryopreservation: occurrence and alleviation. Planta 254:124. DOI: 10.1007/s00425-021-03784-0
Sakai A, Kobayashi S, Oiyama I. 1990. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. Var. Brasiliensis Tanaka) by vitrification. Plant Cell Reports 9:30-33. DOI: 10.1007/BF00232130
Volk GM, Bonnart RM, Araujo De Oliveira A, Henk AD. 2022. Minimizing the deleterious effects of endophytes in plant shoot tip cryopreservation. Applications in Plant Sci. Article e11489. DOI: 10.1002/aps3.11489.
Volk GM, Harris JL, Rotindo KE. 2006. Survival of mint shoot tips after exposure to cryoprotectant solution components. Cryobiology 52:305-308. DOI: 10.1016/j.cryobiol.2005.11.003
Volk GM, Shepherd AN, Bonnart R. 2018. Successful cryopreservation of Vitis shoot tips: novel pre-treatment combinations applied to nine species. CryoLetters 39:322-330. PMID: 30963164
Volk GM, Walters C. 2003. Preservation of genetic resources in the National Plant Germplasm Clonal Collections. Plant Breeding Reviews 23:291-344. DOI: 10.1002/9780470650226.ch7
Volk GM, Walters C. 2006. Plant vitrification solution 2 lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 52:48-61. DOI: 10.1016/j.cryobiol.2005.09.004
Wang M-R, Chen L, da Silva JAT, Volk GM, Wang Q-C. 2018. Cryobiotechnology of apples (Malus spp.): recent and future prospects. Plant Cell Reports 37:689-709. DOI: 10.1007/s00299-018-2249-x
Zhang A-L, Wang M-R, Li Z, Panis B, Bettoni JC, Vollmer R, Xu L, Wang Q-C. 2023. Overcoming Challenges for Shoot Tip Cryopreservation of Root and Tuber Crops. Agronomy 13:219. DOI: 10.3390/agronomy13010219
7. Acknowledgments
Chapter citation: Bettoni JC, Chen K, Volk GM. 2024. An Overview of Shoot Tip Cryopreservation. In: Volk GM (Eds.) Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules. Fort Collins, Colorado: Colorado State University. Date accessed. https://colostate.pressbooks.pub/clonalcryopreservation/chapter/an-overview-of-shoot-tip-cryopreservation/
This project was funded by the USDA-ARS and by the USDA-NIFA Higher Education Challenge Program grant 2020-70003-30930, with support for associated videos from IMLS Grant # MG-70-18-0063-18 to the Center of Plant Conservation.
USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Formation of an amorphous, glassy state (as opposed to a crystalline structure)