Introduction of Clean Plants into Tissue Culture: Temperate Crops
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
Jeanine DeNoma, USDA-ARS National Clonal Germplasm Repository, 33447 Peoria Road, Corvallis, Oregon 97333.
Kim Hummer, USDA-ARS National Clonal Germplasm Repository, 33447 Peoria Road, Corvallis, Oregon 97333.
Katheryn Chen, Department of Soil and Crop Sciences, Colorado State University, 307 University Ave., Fort Collins, Colorado 80523.
Outline
- Introduction to plant tissue culture and its value for genebank conservation
- Selection of plants for introduction into tissue culture
- Surface sterilization
- Endophyte assessment
- Tissue culture establishment, subculture, and multiplication
- References
- Additional information
- Acknowledgments
1. Introduction to plant tissue culture and its value for genebank conservation
Some genebank collections are maintained in tissue culture, which may allow them to be more easily distributed and backed-up at a secondary location. These collections require personnel to maintain the cultures and may be susceptible to somaclonal variation (Bairu et al., 2011). In some cases, collections can be placed into reduced-temperature storage to prolong the times between culture transfers, as described in the chapter “Reduced-Temperature Storage of Temperate Crops in Tissue Culture“. Often, plants are introduced into tissue culture from field, greenhouse, or screenhouse conditions. These plants may have surface contamination or endophytic contamination that should be eliminated during the introduction process.
This chapter describes and demonstrates methods that have been successful for introducing clean mint and strawberry plants from greenhouse conditions into tissue culture. USDA National Plant Germplasm Collections for both of these crops are maintained in greenhouses at the National Clonal Germplasm Repository (NCGR) in Corvallis, Oregon. Tissue cultures of these materials have been maintained in reduced-temperatures for medium-term storage, and have also provided the source plants for shoot tip cryopreservation, as described in the chapter “Mint Shoot Tip Cryopreservation“.
2. Selection of plants for introduction into tissue culture
It is important to select plant materials for introduction into tissue culture that are most likely to be free of endophytes and surface contamination. It is generally preferable to use source plants that have young, actively growing tissue in greenhouse or screenhouse conditions (Fig. 1, 2). Alternate source material, such as young plant tissue from the field or even cuttings of winter budwood that are forced indoors, may be substituted if healthy greenhouse or screenhouse material is not available.
Actively growing shoots or runners of greenhouse-grown plants are harvested with tools that are dipped in 20% bleach (1.2% sodium hypochlorite) and rinsed with water between each cut. They are then trimmed with fine scissors; leaves are removed and plant shoots are cut into lengths of 4-5 cm (Fig. 3). The trimmed shoots are kept in beakers of water to keep them hydrated until all the plant materials are collected.
Figure 1. Greenhouse-grown mint plants are source material for shoots that will be introduced into tissue culture (left). A mint shoot, from which tissue culture explant will be taken (right). Photo credit: Jeanine DeNoma.
Figure 2. Greenhouse-grown strawberry plants serve as materials for introduction into tissue culture (left). A shoot is taken from a strawberry runner (right). Photo credit: Jeanine DeNoma.
Figure 3. Supplies for greenhouse plant harvest include 20% bleach (1.2% sodium hypochorite) to disinfect tools between each shoot, water to rinse tools, fine scissors to cut shoot tips, and labeled beakers with water to keep the shoot tips hydrated (left). Larger untrimmed and trimmed strawberry shoots are shown (right). Photo credit: Jeanine DeNoma.
3. Surface sterilization
In the laboratory, the beakers with water and plant shoots are covered in cheesecloth and washed under running water for 10 minutes (Fig. 4). Multiple accessions can be processed by placing beakers in a tub with running water. Next, the shoot tips are submerged in 10% bleach (0.6% sodium hypochlorite) and placed on a shaker for 10 minutes. The bleach for this protocol contains 6% sodium hypochlorite; however, a more concentrated bleach may be substituted if diluted to an equivalent concentration of sodium hypochlorite. Do not substitute “splashless” bleach. If contamination is a concern, a drop of Tween 20 can be added to the bleach solution. Ensure that there are no air pockets and that the bleach makes contact with the surface of the shoot tips (Fig. 4).
After the ten-minute bleach treatment, the beaker is transferred to a laminar flow hood and the shoots are transferred to sterile water. Swirl the shoots in the water to rinse well. Shoots are then transferred to a second flask of sterile water and swirled to ensure that the bleach is rinsed off the shoots (Fig. 5). Up to six beakers of shoots can be processed at a time.
Figure 4. Shoot tips in a beaker are rinsed with tap water for 10 minutes (left). The subsequent surface sterilization step requires 10% bleach with Tween 20 and sterile rinse water (middle). Shoot tips are paced in the bleach on shaker for 10 minutes (right). Photo credit: Jeanine DeNoma.
Figure 5. Shoots are transferred into a laminar flow hood (left). After 10 minutes in the bleach solution, shoot tips are rinsed twice in sterile water (right). Photo credit: Jeanine DeNoma.
4. Endophyte assessment
Some plants are hosts for a wide range of endophytes, which may result in contamination when propagules are placed on a nutrient-rich, high-sugar medium. This section demonstrates a screening process by which plant shoots are incubated on a medium to determine if they are contaminated (Buckley et al., 1995). This example uses Nutrient Agar medium (8 g L-1 nutrient broth + 1 g L-1 yeast extract + 10 g L-1 glucose + 8 g L-1 agar) as a substrate to identify endophyte growth.
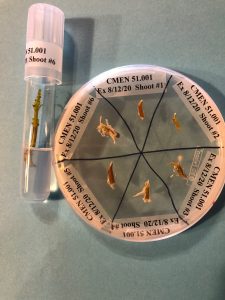
Within a laminar flow hood, cut a 2 mm basal portion from each surface-sterilized shoot using a sterile tile or large Petri dish as a cutting surface (Fig. 6). Tools should be sterilized between shoots to avoid cross-contamination. The basal portions are placed on labeled sections of a Nutrient Agar plate. The larger portions, which include the shoot tip, are each placed into a test tube of propagation medium (Reed et al., 1995).
Figure 6. The supplies needed to test shoots for endophytes include surface sterilized plant shoots, a divided plate of Nutrient Agar, and individual tubes of propagation medium (left). Basal portions are removed from each shoot (right) and then placed onto Nutrient Agar. Photo credit: Jeanine DeNoma.
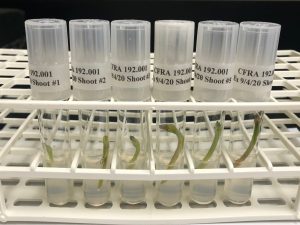
The shoot segments on the Nutrient Agar plates and on the propagation medium are both placed under low light in the growth room for three weeks, at which time the shoots on the propagation medium are transferred to a fresh medium. The cultures are kept at 25 oC in a 16-h photoperiod at approximately 5-10 µmol m-2 s-1 photo-synthetic photon flux (PAR). The shoots on both Nutrient Agar and on propagation medium are regularly checked for contamination (Fig. 7, 8, 9). If a segment on Nutrient Agar is contaminated, the corresponding segment on propagation medium is autoclaved and discarded. If the segment on propagation medium is contaminated, it is autoclaved and discarded.
In some cases, all of the shoots introduced into tissue culture exhibit contamination on either the Nutrient Agar or multiplication medium. In this case, plants can be re-introduced from a cleaner or alternative source plant. It may also be necessary to grow plants on a medium that contains a broad-spectrum biocide designed for tissue culture, such as Plant Preservation Mixture (PPM). PPM targets both bacteria and fungi; the application concentration may have to be optimized for the specific crop. Alternatively, antibiotics may be applied to eliminate the endophytic bacteria. The specific antibiotic(s) needed depends on the susceptibility of the endophyte and tolerance levels of the plant material (Reed et al., 1995; Reed and Tanprasert, 1995; Tanprasert and Reed, 1997). New genomic techniques may be helpful for endophyte identification and antibiotic selection (Tekielska et al., 2019).
Figure 7. Mint shoot tips are incubated on Nutrient Agar medium for up to three weeks in a growth room to determine if endophytes are present. Any explants on culture medium that correspond to contaminated explants on Nutrient Agar medium are discarded. In this example, Explant #4 and Explant #6 show contamination and the corresponding shoots on propagation medium are discarded. Photo credit: Jeanine DeNoma.
Figure 8. Surface sterilized strawberry shoots are cut into a small basal section and a longer shoot section, and instruments are sterilized after each explant is handled. The basal section of each explant is placed onto Nutrient Agar medium in a Petri dish (left) and the corresponding shoot is placed onto propagation medium in test tubes (right). Photo credit: Jeanine DeNoma.
Figure 9. In this example, strawberry Explant #1 on Nutrient Agar exhibits contamination, so the corresponding shoot on culture medium will be discarded. Photo credit: Jeanine DeNoma.
5. Tissue culture establishment, subculture, and multiplication
Clean plants are maintained on propagation medium. The plants grow taller and multiple shoots may form. After a few weeks or months of culture in the growth room at 25 oC, 16-h photoperiod, 25-60 µmol m-2 s-1 photo-synthetic photon flux (PAR), the plants can be removed from their containers in a laminar flow hood and cut into pieces with one or more nodes. These pieces are then placed onto a fresh medium. As a result, many identical plants can be grown from the initial plant in a process called “subculturing”. Examples of mint and strawberry subculturing are shown below.
The first example shows the subculture process for mint. A sterile cube containing several mint plants is sprayed with 70% ethanol and introduced into a laminar flow hood (Fig . 10). Within the laminar flow hood, the container lid is removed. An individual plant is transferred to a glass Petri dish and then cut into nodal sections with a scalpel with a sterile blade (Fig. 11). Leaves may optionally be removed and the nodal section is placed onto fresh medium (Fig. 12). The process is shown in Video 1.
Figure 10. In vitro mint plant that will be subcultured to produce additional plants. Photo credit: Katheryn Chen.
Figure 11. In a sterile environment, a mint shoot is removed from the culture vessel and cut into single node sections. Photo credit: Katheryn Chen.
Figure 12. Leaves may be removed before the nodal section is placed onto fresh culture medium (left). In this example, five nodal sections are put into one culture vessel (right). Photo credit: Katheryn Chen.
Video 1. Technician Katheryn Chen (Colorado State University) demonstrates the process of subculturing mint. (silent video)
The second example shows a similar process to subculture strawberry. A cube of strawberry plants is sprayed with 70% ethanol and placed into a laminar flow hood. Strawberry plants often grow as clumps (Fig. 13). A group of plants is removed from the cube and placed onto a sterile Petri dish. Individual plants are separated from the others, and leaves may optionally be removed (Fig. 14). The resulting plants are placed onto fresh medium (Fig. 15). The process is demonstrated in Videos 2 and 3.
Figure 13. In vitro strawberry plant that will be subcultured to produce additional plants. Photo credit: Jeanine DeNoma.
Figure 14. In a sterile environment, strawberry plant clump is removed from the culture vessel and placed onto a sterile Petri dish (left). Individual plantlets are separated and leaves may optionally be removed (right). Photo credit: Katheryn Chen.
Figure 15. The plantlet is then placed onto fresh culture medium (left). In this example, five nodal sections are put into one culture vessel (right). Photo credit: Katheryn Chen.
Video 2. Technician Katheryn Chen demonstrates the process of subculturing strawberry. (silent video)
Video 3. Technician Jeanine DeNoma demonstrates the process of dividing strawberry cultures prior to shoot-tip cryopreservation. (silent video)
6. References
Bairu MW, Aremu AO, Van Staden J. 2011. Somaclonal variation in plants: causes and detection methods. Plant Growth Regulation 63:147–173.
Buckley PM, DeWilde TN, Reed BM. 1995. Characterization and identification of bacteria isolated from micropropagated mint plants. In Vitro Cellular and Developmental Biology 31:58-64.
Reed BM, Buckley PM, DeWilde TN. 1995. Detection and eradication of endophytic bacteria from micropropagated mint plants. In Vitro Cellular and Developmental Biology 31:53-57.
Reed BM, Tanprasert P. 1995. Detection and control of bacterial contaminants of plant tissue cultures. A review of recent literature. Plant Tissue Culture and Biotechnology 1:137-142.
Tanprasert P, Reed BM. 1997. Determination of minimal bactericidal and effective antibiotic treatment concentrations for bacterial contaminants from micropropagated strawberries. In Vitro Cellular and Developmental Biology 3:227-230.
Tekielska D, Peňázová E, Kovács T, Křižan B, Čechová J, Eichmeier A. 2019. Bacterial contamination of plant in vitro cultures in commercial production deteted by high-throughput amplicon sequencing. Acta Universitatis Agricultrae et Silviculturae Mendelianae Brunensis 67:1005-1014.
7. Associated information
PPM is provided by Plant Cell Technology.
8. Acknowledgments
Citation: Volk GM, DeNoma J, Hummer K, Chen K. 2021. Introduction of clean mint and strawberry plants into tissue culture. In: Volk GM (Eds.) Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules. Fort Collins, Colorado: Colorado State University. Date accessed. Available from: https://colostate.pressbooks.pub/clonalcryopreservation/chapter/introduction-of-plants-into-tissue-culture/
This training module was made possible by:
Editors: Gayle Volk, Katheryn Chen
Content providers: Jeanine DeNoma, Katheryn Chen, Kim Hummer
Methods developed by: Barbara Reed
This project was funded by the USDA-ARS and and by the USDA-NIFA Higher Education Challenge Program grant 2020-70003-30930.
USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.