Cryotherapy Using Shoot Tip Cryopreservation
Jean Carlos Bettoni, Independent Researcher, 35 Brasil Correia Street, Videira, SC 89560510, Brazil. jcbettoni@gmail.com
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
The purpose of this chapter is to describe the concept of shoot tip cryotherapy—the application of shoot tip cryopreservation to eradicate pathogens.
Outline
- Introduction
- Principles of shoot tip cryotherapy
- Development of shoot tip cryotherapy procedures
- Regrowth and pathogen detection
- Efficacy of cryotherapy
- Conclusions
- References
- Acknowledgments
1. Introduction
Most fruit and nut cultivars, as well as some root and tuber vegetables and sugar crops, are vegetatively propagated to maintain their specific genetic combinations. New plants that are identical to the initial plant are produced by grafting, rooted cuttings, runners, rhizomes, tubers, etc. (Figure 1). If stock plants harbor diseases, those pathogens might be transmitted to the offspring when infected materials are vegetatively propagated, and this represents a major constraint for crop production. Infected plants usually have reduced yields and an increased susceptibility to other pathogens. Moreover, the presence of pathogens limits the safe movement of plant materials across borders. Propagating clean, pathogen-free stock plants is the most effective and sustainable strategy to control the presence and spread of viruses in the field. Improved methods for producing disease-free plants are therefore needed to control the spread of pathogens (Rubio et al., 2020; Barba et al., 2017).
 Figure 1. Examples of propagation methods: A) apple grafting, B) strawberry runners, C) garlic bulbs, D) potato tubers. Photo credits: Remi Bonnart (A), G. Volk (B,C), and USDA (D).
Figure 1. Examples of propagation methods: A) apple grafting, B) strawberry runners, C) garlic bulbs, D) potato tubers. Photo credits: Remi Bonnart (A), G. Volk (B,C), and USDA (D).
Tender 1–2 mm shoot tips can survive liquid nitrogen (LN) storage if processed with optimized cryopreservation protocols. When the shoot tips are derived from infected plants, a percentage of the plants recovered after LN exposure will not harbor the pathogens that were in the source plant. This process of eliminating pathogens is termed cryotherapy. Cryotherapy has been used to eliminate viruses, viroids, phytoplasma, and bacteria from plant materials. Shoot tip cryotherapy is transitioning from primarily a research effort to a more prevalent method in pathogen elimination operations (Wang et al., 2022). Detailed information about shoot tip cryotherapy for pathogen eradication can be found in multiple reviews (Wang and Valkonen, 2009; Zhao et al., 2019; Bettoni et al., 2021; Wang et al., 2022).
2. Principles of shoot tip cryotherapy
Shoot tip-based pathogen elimination methods depend on pathogens preferentially residing in certain cells. Many pathogens, including most viruses, are absent from the undifferentiated cells in the apical dome of the meristem. Cells in the apical meristems are smaller in size, have smaller vacuoles, and a higher nucleocytoplasmic ratio than the cells in more differentiated tissues (Figure 2). Most pathogens are typically found in differentiated plant cells, particularly the phloem. In addition, phytoplasmas and gram-negative bacteria inhabit vascular tissues and cannot invade the meristem (Hogenhout et al., 2008). The pathogen location and the differential survival of cells as a result of cryoexposure are key to the success of cryotherapy.
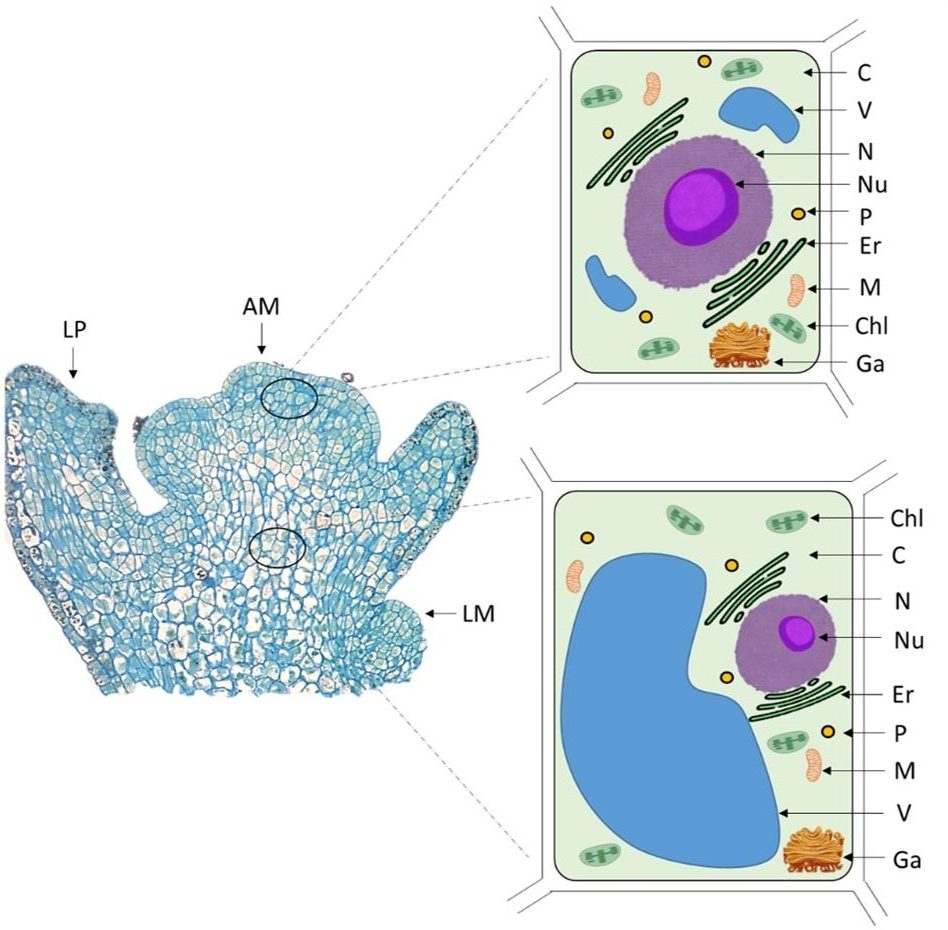 Figure 2. Schematic illustration of some cell types present within plant shoot tips. Excised shoot tips include the apical meristem (AM), leaf primordia (LP), and cells that are undergoing differentiation, such as protophloem and protoxylem. Cells in the AM are smaller and have smaller vacuoles and a higher nucleocytoplasmic ratio than cells in the more differentiated tissues. AM, apical meristem; C, cytoplasm; Chl, chloroplast; Er, endoplasmic reticulum; Ga, Golgi apparatus; M, mitochondria; LM, leaf meristem; LP, leaf primordium; N, nucleus; Nu, nucleolus; P, peroxisome; V, vacuole. Figure by JC Bettoni.
Figure 2. Schematic illustration of some cell types present within plant shoot tips. Excised shoot tips include the apical meristem (AM), leaf primordia (LP), and cells that are undergoing differentiation, such as protophloem and protoxylem. Cells in the AM are smaller and have smaller vacuoles and a higher nucleocytoplasmic ratio than cells in the more differentiated tissues. AM, apical meristem; C, cytoplasm; Chl, chloroplast; Er, endoplasmic reticulum; Ga, Golgi apparatus; M, mitochondria; LM, leaf meristem; LP, leaf primordium; N, nucleus; Nu, nucleolus; P, peroxisome; V, vacuole. Figure by JC Bettoni.
While pathogen elimination through traditional shoot tip culture depends on precisely excising tiny shoot tips (0.3–0.5 mm) that exclude any infected cells, shoot tip cryotherapy uses larger shoot tips (1–2 mm) and relies on the differential survival of infected versus pathogen-free cells. When optimized methods are used to expose shoot tips to LN, as described in the chapter “An Overview of Shoot Tip Cryopreservation“, the meristem cells will survive while the vacuolated and differentiated cells that can harbor pathogens usually do not survive. As a result, healthy and disease-free plants can be regenerated.
3. Development of shoot tip cryotherapy procedures
Cryopreservation protocols have been developed for a wide range of plant species. In some cases, cryopreservation procedures might need adjustments that decrease survival of non-meristematic cells in order to increase the frequency of pathogen eradication by cryotherapy. Variables that can be altered to increase cell mortality include pretreatments, durations, and conditions of exposure to plant vitrification solutions or air dehydration. Multiple shoot tip cryopreservation methods have been tested for virus eradication; both encapsulation-dehydration and vitrification-based methods can effectively eliminate viruses.
In 1997, Brison et al. (1997) reported the first successful eradication of Plum pox virus from the infected shoot tips of a Prunus rootstock. Since then, the list of successfully eradicated pathogens has grown and the technology has become more widely adopted in many economically important vegetatively propagated crops, including root, bulb, and tuber vegetable crops, sugar crops, nuts, fruits, and berries. A few examples include potato (Figure 3), garlic, apple, and grapevine (Wang et al., 2022).
 Figure 3. Potato in vitro plant recovered after cryotherapy. Photo credit: J.C. Bettoni.
Figure 3. Potato in vitro plant recovered after cryotherapy. Photo credit: J.C. Bettoni.
In some cases, it is advantageous to combine pathogen elimination methods. Thermotherapy and chemotherapy treatments reduce virus titers and produce larger virus-free areas in the apical meristem, enhancing virus eradication following shoot tip cryotherapy. Additionally, thermotherapy induces structural changes in shoot tip cells (enlarged vacuoles), which can increase their susceptibility to cryo-injury in infected differentiated cells and, eventually improve virus eradication frequency (Wang et al., 2018).
4. Regrowth and pathogen detection
Successful cryotherapy depends on the availability of an effective recovery system. Optimized regrowth conditions are essential to produce direct shoot development without intermediate callus formation. In addition, proper handling methods are crucial to avoid cross-contamination during the recovery process.
Standard procedures for detecting specific pathogens usually determine if the pathogen was successfully eliminated by cryotherapy. Sensitive detection systems ensure correct diagnosis of the pathogen status. Understanding the disease cycle of pathogens and crops is important to guard against possible false negatives. The sanitary status of plants that undergo therapies should not rely just on the test result for in vitro plants, especially in woody plant species (Figure 4). In vitro plants that initially show negative results might later show positive results in vivo, because low-titer pathogens are not always detected by some testing methods (Rubio et al., 2020). Consequently, pathogen testing is often performed after plants are transferred to soil in a growth chamber or greenhouse environment and also after a dormancy period, if applicable (Figure 5).
 Figure 4. Apple shoot tips regenerating after cryotherapy (left), and establishing healthy in vitro cultures (right). Photo credit: J.C. Bettoni.
Figure 4. Apple shoot tips regenerating after cryotherapy (left), and establishing healthy in vitro cultures (right). Photo credit: J.C. Bettoni.
 Figure 5. Apple plants that underwent cryotherapy, recovered in vitro, and then transferred to soil. Photo credit: J.C. Bettoni.
Figure 5. Apple plants that underwent cryotherapy, recovered in vitro, and then transferred to soil. Photo credit: J.C. Bettoni.
5. Efficacy of cryotherapy
During development of cryotherapy methods, the percentage of clean plants that are recovered after cryotherapy procedures must be determined. This is a comparison of the number of shoot tips initially excised and the number of shoot tips that were successfully regenerated into clean plants. This information is required to calculate how many shoot tips should be exposed to LN for each stock plant that undergoes therapy. The likely variation among diverse cultivars should also factor into these calculations. Only one clean, healthy plant is necessary because that plant can be clonally propagated to produce many more disease-free plants.
The range of efficacy for cryotherapy reported in the literature appears to depend on the type of pathogen and plant species/genotype. Shoot tip cryotherapy often fails to eradicate pathogens that infect meristematic cells. Hence, a small portion of the infected cells could remain alive following LN exposure, causing the regenerated plants to remain diseased. Improvements in pathogen eradication, especially for pathogens that penetrate meristematic cells, have been achieved by combining thermotherapy or chemotherapy with cryotherapy (Figure 6) (Bettoni et al., 2022; Zhao et al., 2019).
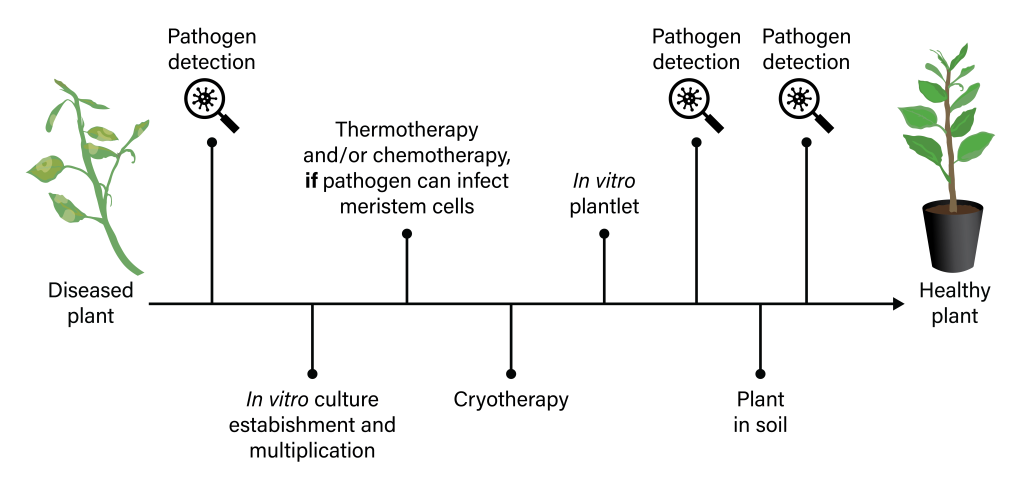 Figure 6. Simplified workflow for the production of pathogen-free plants. Techniques are applied either alone or in combination and the selection of a specific pathogen eradication method is related to plant-virus interactions and in planta distribution patterns. Figure by K. Chen.
Figure 6. Simplified workflow for the production of pathogen-free plants. Techniques are applied either alone or in combination and the selection of a specific pathogen eradication method is related to plant-virus interactions and in planta distribution patterns. Figure by K. Chen.
6. Conclusions
It is relatively easy for laboratories that routinely perform traditional meristem culture to implement cryotherapy for pathogen eradication, provided that methods are available. When compared with the excision process used in traditional shoot tip culture methods, excising larger shoot tips for cryotherapy significantly improves the rate at which technicians can produce quality shoot tips, thereby increasing germplasm survival and regrowth, as well as overall operational efficiency (Wang and Valkonin, 2009; Bettoni et al., 2021; Wang et al., 2022). Precautions are needed to avoid cross contamination with clean materials, which may require specific biosafety level laminar flow hoods and ventilation systems.
In some cases, such as when a virus infects meristematic cells, the combination of thermotherapy/chemotherapy and cryotherapy might be the only way to successfully eliminate pathogens. However, when cryotherapy alone can eradicate a pathogen, its capacity for processing a large number of shoot tips quickly and simultaneously can reduce costs and provide a significant advantage when compared to traditional thermotherapy or chemotherapy followed by shoot tip culture.
There are many opportunities for cryotherapy applications to provide plant certification programs a supply of healthy plant materials for international exchanges as well as to release clean materials to the nursery industry (Bettoni et al., 2022).
7. References
Barba M, Hosakawa M, Wang Q-C, Taglienti A, Zhang Z. 2017. Viroid elimination by thermotherapy, cold therapy, tissue culture, in vitro micrografting, or cryotherapy. Academic Press, Oxford, United Kingdom. DOI: 10.1016/B978-0-12-801498-1.00040-1
Bettoni JC, Fazio G, Carvalho Costa L, Hurtado-Gonzales OP, Rwahnih MA, Nedrow A, Volk GM. 2022. Thermotherapy followed by shoot tip cryotherapy eradicates latent viruses and apple hammerhead viroid from in vitro apple rootstocks. Plants 11:582. DOI: 10.3390/plants11050582
Bettoni JC, Marković Z, Bi W, Volk GM, Matsumoto T, Wang Q-C. 2021. Grapevine shoot tip cryopreservation and cryotherapy: secure storage of disease-free plants. Plants 10:2190. DOI: 10.3390/plants10102190
Brison M, Boucaud MT, Pierronnet A, Dosba, F. 1997. Effect of cryopreservation on the sanitary state of a cv. Prunus rootstock experimentally contaminated with Plum pox potyvirus. Plant Science 123:189-196. DOI: 10.1016/S0168-9452(97)04581-0
Hogenhout SA, Oshima K, Ammar ED, Kakizawa S, Kingdom HN, Namba S. 2008. Phytoplasmas: bacteria that manipulate plants and insects. Molecular Plant Pathology 9:403-423. DOI: 10.1111/j.1364-3703.2008.00472.x
Rubio L, Galipienso L, Ferriol I. 2020. Detection of plant viruses and disease management: relevance of genetic diversity and evolution. Frontiers in Plant Science 11:1092. DOI: 10.3389/fpls.2020.01092
Wang M-R, Bi W-L, Bettoni JC, Zhang D, Volk GM, Wang Q-C. 2022. Shoot tip cryotherapy for plant pathogen eradication. Plant Pathology 71:1241-1254. DOI: 10.1111/ppa.13565
Wang M-R, Cui Z-H, Li J-W, Hao X-Y, Zhao L, Wang Q-C. 2018. In vitro thermotherapy-based methods for plant virus eradication. Plant Methods 14:87. DOI: 10.1186/s13007-018-0355-y
Wang Q, Valkonen JPT. 2009. Cryotherapy of shoot tips: novel pathogen eradication method. Trends in Plant Science 14:119-122. DOI: 10.1016/j.tplants.2008.11.010
Zhao L, Wang MR, Li J, Cui Z, Volk GM, Wang Q. 2019. Cryobiotechnology: a double-edged sword for obligate plant pathogens. Plant Disease 103:1058-1443. DOI: 10.1094/PDIS-11-18-1989-FE
8. Acknowledgments
Chapter citation: Bettoni JC, Volk GM. 2024. Cryotherapy Using Shoot Tip Cryopreservation. In: Volk GM (Eds.) Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules. Fort Collins, Colorado: Colorado State University. Date accessed. Available from https://colostate.pressbooks.pub/clonalcryopreservation/chapter/cryotherapy-using-shoot-tip-cryopreservation/
Chapter editor: Katheryn Chen
This project was funded by the USDA-ARS and by the USDA-NIFA Higher Education Challenge Program grant 2020-70003-30930.
USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.


