Considerations When Implementing Shoot Tip Cryopreservation
Jean Carlos Bettoni, Independent Researcher, 35 Brasil Correia Street, Videira, SC 89560510, Brazil. jcbettoni@gmail.com
Katheryn Chen, Department of Soil and Crop Sciences, Colorado State University, 307 University Ave., Fort Collins, Colorado 80523.
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
The purpose of this chapter is to present some considerations when implementing shoot tip cryopreservation procedures within cryobanks.
Outline
- Introduction
- Prioritizing materials
- Selecting cryopreservation methods
- Long-term storage
- Documentation and organization
- Additional resources
- References
- Acknowledgments
1. Introduction
Shoot tip cryopreservation is a complicated process that requires thoughtful consideration at every step in order to successfully preserve germplasm for future use. In addition to short term funding, cryopreservation programs depend on the stability and longevity provided by long-term planning and funding. Before any material is cryopreserved, programs must establish infrastructure, hire skilled technical support staff, identify conservation goals, target appropriate accessions and source plants, and evaluate existing preservation methodologies. When protocols are available, it is critical to determine if the methods are widely applicable to the diversity within the crop that will be cryopreserved; otherwise, modifications might be needed. Before transitioning into large-scale production, it is important to establish clear levels of acceptable standard viabilities based on shoot regrowth. Data management and labeling practices should also be standardized at this point. All protocols must be clearly documented so that future generations can repeat the procedures and successfully regenerate a normal plant when needed (Bettoni et al., 2021).
2. Prioritizing materials
Many factors must be considered when prioritizing accessions for cryopreservation. These decisions should ideally be made by a multidisciplinary group including the curation teams, crop experts including breeders, and the cryopreservation scientist. The interdisciplinary team can identify and prioritize accessions in collections, confirm redundancies and verify uniqueness, and consequently increase efficiency of collection preservation efforts (Bramel and Volk, 2019).
 Figure 1. Apple Crop Germplasm Committee meeting in Geneva, New York, USA. Photo credit: G. Volk.
Figure 1. Apple Crop Germplasm Committee meeting in Geneva, New York, USA. Photo credit: G. Volk.
Cryopreserving plant accessions that might offer both genetic and phenotypic novelty to the collection and are being threatened in natural environments by biotic and abiotic stresses are often prioritized for cryopreservation. Given the amount of resources that will be invested in the process of cryopreserving plant collections as shoot tips, it is best to prioritize plants with verified identities. Accessions with high-quality, pathogen-free source plants are more common targets for large-scale operations as they are more likely to survive the cryopreservation process.
In some cases, clonal cryopreservation might not be the most effective strategy to secure field and greenhouse collections. It may be more efficient to make controlled crosses and collect the allelic diversity within a population or species as seeds—especially when the seeds are orthodox types (Walters et al., 2008). When clonal individuals are targeted, dormant buds might be preferred if proven cryopreservation methods are available. Although comparatively few crops have been successfully cryopreserved as dormant buds, their preservation process in a large-scale operation is significantly faster and less resource-intensive than is shoot tip preservation (Tanner et al., 2021).
3. Selecting cryopreservation methods
Shoot tip cryopreservation methods vary widely within and among species. As a first step in choosing a specific protocol, the current literature available on the previous cryopreservation successes in similar or related crops should be analyzed. Some practical and useful resources on methods for shoot tip cryopreservation can be found in recent review papers (Zhang et al., 2023; Vollmer et al., 2022; Bettoni et al., 2021; Niino et al., 2019; Wang et al., 2018; Matsumoto, 2017; Sakai and Engelmann, 2007), practical guides (Reed, 2008), and in this eBook.
Often, more than a single method is suitable for preservation of a selected species (Figure 2). Selecting a method depends on the crop, available equipment, technical skills, budget, and project goals (Bettoni et al., 2021). Therefore, each laboratory must determine which method is most effective under their conditions. When several options are possible, priority should be given to the cryopreservation method that requires the least input of time and resources and that is effective for a wide range of cultivars and even species.
 Figure 2. Droplet-vitrification (left) and encapsulation-dehydration (right) shoot tip cryopreservation protocols. Photo credit: K. Chen.
Figure 2. Droplet-vitrification (left) and encapsulation-dehydration (right) shoot tip cryopreservation protocols. Photo credit: K. Chen.
Cryopreservation protocols are typically developed and optimized with a few accessions and sometimes these protocols are not necessarily applicable to the wide range of cultivars and wild species that are represented in crop collections. When conserving a wide range of genotypes that respond differently to a protocol, modifications to the methods, or multiple different methods might be needed.
4. Long-term storage
The quantity of stored material depends on infrastructure, availability of technical staff, material availability, and preservation goals. The total number of stored propagules should be high enough to be able to produce sufficient quantities of living plants to meet any future needs, but ideally not so high as to place undue burden on staff or resources. The number of propagules per vessel should also be a consideration; a vial should ideally contain as many shoot tips as is appropriate for a single regeneration event, so that the total number of vials stored represents the number of opportunities for future regeneration.
The USDA-ARS National Plant Germplasm System’s National Laboratory for Genetic Resources Preservation (NLGRP), in Fort Collins, Colorado, USA (Figure 3), aims to achieve a minimum of 40% viability and 60 predicted viable propagules each time an accession is processed. A 40% viability is selected as the standard because it provides a 95% probability that at least 1 of the 10 shoot tips in each vial is alive (Volk et al., 2017). Currently at NLGRP, clonal cryopreservation procedures target a total of 170–180 shoot tips per accession; 20 of these are used shortly after cryoexposure for viability testing, while the remaining 150–160 are conserved for long-term storage, provided that the minimum 40% viability standard is met. Accessions with zero viability are discarded. Those with poor viabilities may be saved until they are successfully cryopreserved at a later date using either the same, a slightly modified, or a completely different protocol. In some cases, vials from multiple cryopreservation events may be stored to increase the total number of predicted viable propagules in storage.
 Figure 3. Cryotank storage at the USDA-ARS National Laboratory for Genetic Resources Preservation (NLGRP), in Fort Collins, Colorado. Photo credits: Jennifer Kendall.
Figure 3. Cryotank storage at the USDA-ARS National Laboratory for Genetic Resources Preservation (NLGRP), in Fort Collins, Colorado. Photo credits: Jennifer Kendall.
Although it is not a common problem in well-maintained genebanks, any cryotank can fail and thereby jeopardize the germplasm it stores. Semi-automated liquid nitrogen filling systems help to reduce the chance of low liquid levels or warming in cryotanks, however, manually-filled tanks have fewer points of possible failure and might last longer. Regardless of tank age and type, it is beneficial to create safety-duplicates whenever possible in order to minimize the chance of loss; this can be achieved simply by splitting an inventory across two or more cryotanks.
5. Documentation and organization
For long-term storage, it is necessary to focus on future use of the germplasm, ensuring predictability and reliability. Well-defined operational protocols and guidelines must be documented to ensure the integrity and consistency of all information associated with the cryopreservation method, storage conditions, and recovery techniques. High quality documentation is needed at every step of the process and is critical not only for internal organization and use, but also to reduce procedural misinterpretations when knowledge is transferred between laboratories. The general activities involving the implementation of shoot tip cryopreservation for long-term storage are represented in Figure 4.
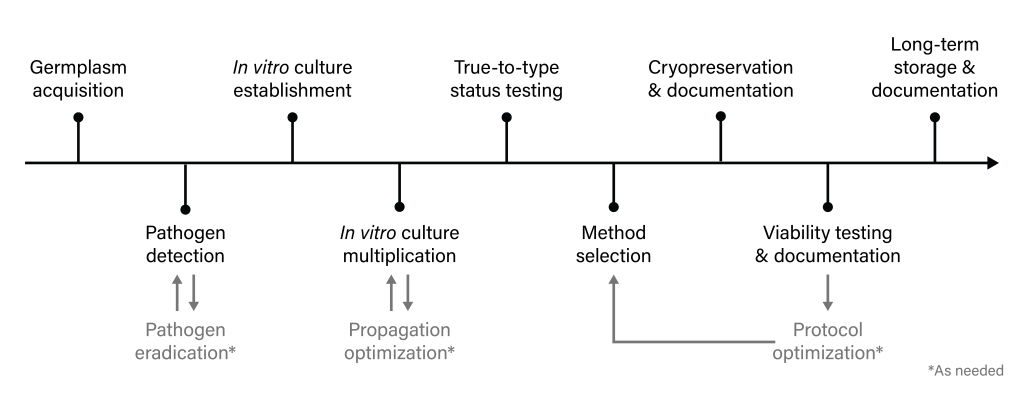 Figure 4. Flowchart depicting general components for implementing shoot tip cryopreservation for long-term storage. Figure by J.C. Bettoni and K. Chen.
Figure 4. Flowchart depicting general components for implementing shoot tip cryopreservation for long-term storage. Figure by J.C. Bettoni and K. Chen.
Accurate labeling and record keeping are essential for the efficient and successful retrieval of accessions when they are needed. There are two principal locations where data must be exact: on the inventory and in the database. The inventory itself must contain a unique identifier that can distinguish it from any other accession or even freeze event. This might be a string of numbers or letters (with or without associated meanings) clearly printed onto a cryogenic label, a one-dimensional barcode, two-dimensional QR code, or a scannable RFID tag. Additional information on the vials such as accession number, taxon, cultivar name, freeze date, and processing technician are not strictly necessary, but can help to improve readability and lessen the chance for human error. Labels elsewhere, including on the tops of cryocanes, cryoboxes or SUC-1 cannisters, or on the tank can help to improve organization, thus facilitating efficient storage and retrieval (Figure 5).
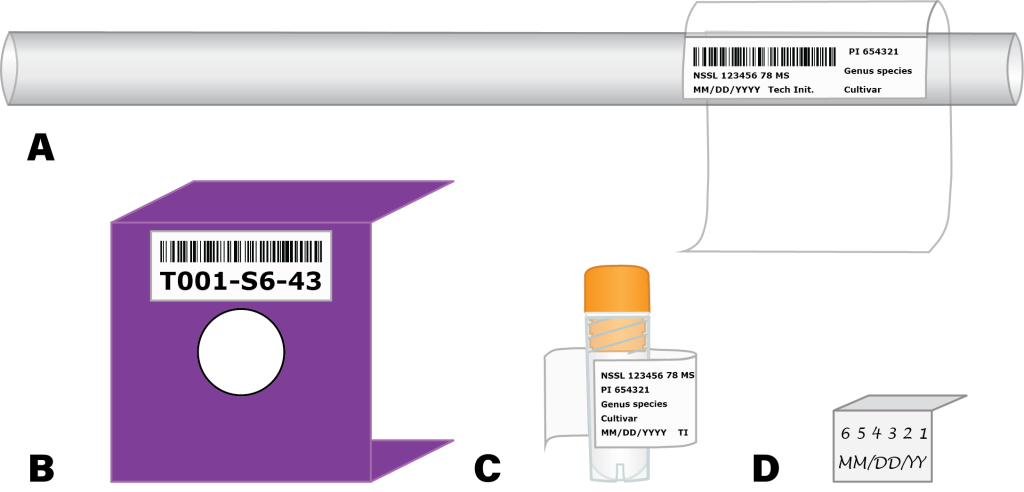 Figure 5. Examples of labeling systems used at the USDA-ARS National Laboratory for Genetic Resources Preservation. A) cryosleeve, B) lid to SUC-1 cryocannister, C) cryovial, D) cryocane ID tab. Figure by K. Chen.
Figure 5. Examples of labeling systems used at the USDA-ARS National Laboratory for Genetic Resources Preservation. A) cryosleeve, B) lid to SUC-1 cryocannister, C) cryovial, D) cryocane ID tab. Figure by K. Chen.
All associated data must be accurately entered into a secure database. For NLGRP and other facilities in the National Plant Germplasm System, this database is GRIN-Global. Records must be able to be traced to the unique identifier for each freeze event of an accession. Data should be entered in a consistent manner and provide detailed information related to storage location, quantity, freeze date, methods, viability results, and more. Data management in these cases can be extremely complex, requiring a data management specialist or team of specialists, as well as written protocols to ensure clarity and continuity over time.
6. Additional resources
For more information on the history, importance, and challenges of clonal cryopreservation, consider viewing this 1 hour webinar, Genebank Resources on the Web Webinar: The Global Plant Cryopreservation Initiative with Dr. David Ellis.
7. References
Bettoni JC, Bonnart R, Volk GM. 2021. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell, Tissue and Organ Culture 144:21-34. DOI: 10.1007/s11240-020-01846-x
Bramel PJ, Volk GM. 2019. A global strategy for the conservation and use of apple genetic resources. Global Crop Diversity Trust, Bonn, Germany. DOI: 10.13140/RG.2.2.34072.34562
Matsumoto T. 2017. Cryopreservation of plant genetic resources: conventional and new methods. Reviews in Agricultural Science 5:13-20. DOI: 10.7831/ras5.13
Niino T, Yamamoto S, Matsumoto T, Engelmann F, Valle Arizaga M, Tanaka D. 2019. Development of V and D cryo-plate methods as effective protocols for cryobanking. Acta Horticulturae 1234:249-262. DOI: 10.17660/ActaHortic.2019.1234.33
Reed BM. 2008. Cryopreservation—Practical considerations. In Plant Cryopreservation: A Practical Guide. Springer, New York, USA. DOI: 10.1007/978-0-387-72276-4_1
Sakai A, Engelmann, F. 2007. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. Cryo Letters 28:151-172. PMID: 17898904
Tanner JD, Chen KY, Bonnart RM, Minas IS, Volk GM. 2021. Considerations for large-scale implementation of dormant budwood cryopreservation. Plant Cell, Tissue and Organ Culture 144:35-48. DOI: 10.1007/s11240-020-01884-5
Volk GM, Henk AD, Jenderek M, Richards CM. 2017. Probabilistic viability calculations for cryopreserving vegetatively propagated collections in genebanks. Genetic Resources and Crop Evolution 64:1613-1622. DOI: 10.1007/s10722-016-0460-6
Vollmer R, Villagaray R, Castro M, Cárdenas J, Pineda S, Espirilla J, Anglin N, Ellis D, Azevedo V. 2022. The world’s largest potato cryobank at the International Potato Center (CIP)—Status quo, protocol improvement through large-scale experiments and long-term viability monitoring. Frontiers in Plant Science 13:1059817. DOI: 10.3389/fpls.2022.1059817
Walters C, Wesley-Smith J, Crane J, Hill LM, Chmielarz P, Pammenter NW, Berjak P. 2008. Cryopreservation of Recalcitrant (i.e. Desiccation-Sensitive) Seeds. In: Reed BM (Eds.) Plant Cryopreservation: A Practical Guide. Springer, New York, USA. DOI: 10.1007/978-0-387-72276-4_18
Wang M-R, Chen L, da Silva JAT, Volk GM, Wang Q-C. 2018. Cryobiotechnology of apples (Malus spp.): recent and future prospects. Plant Cell Reports 37:689-709. DOI: 10.1007/s00299-018-2249-x
Zhang A-L, Wang M-R, Li Z, Panis B, Bettoni JC, Vollmer R, Xu L, Wang Q-C. 2023. Overcoming Challenges for Shoot Tip Cryopreservation of Root and Tuber Crops. Agronomy 13:219. DOI: 10.3390/agronomy13010219
8. Acknowledgments
Chapter citation: Bettoni JC, Chen K, Volk GM. 2024. Considerations When Implementing Shoot Tip Cryopreservation. In: Volk GM (Eds.) Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules. Fort Collins, Colorado: Colorado State University. Date accessed. Available from: https://colostate.pressbooks.pub/clonalcryopreservation/chapter/considerations-when-implementing-shoot-tip-cryopreservation/
This project was funded by the USDA-ARS and by the USDA-NIFA Higher Education Challenge Program grant 2020-70003-30930.
USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.


