Grapevine Shoot Tip Cryopreservation (Droplet Vitrification and V-Cryoplate)
Jean-Carlos Bettoni, The New Zealand Institute for Plant & Food Research Limited, Private Bag 11 600, Palmerston North 4442, New Zealand.
Remi Bonnart, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521.
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
Outline
- Introduction
- Shoot tip excision
- Shoot tip processing and liquid nitrogen exposure
- Long-term storage
- Viability assessment
- References
- Additional information
- Acknowledgments
The Vitis shoot tip droplet vitrification cryopreservation procedure is available for download here.
The Vitis shoot tip regrowth procedure after droplet vitrification is available for download here.
1. Introduction
The USDA-ARS National Plant Germplasm System has two field collections of grapevine (Vitis). A total of 3649 unique cultivars of Vitis are maintained as vines at the National Clonal Germplasm Repository for Tree Fruits and Nut Crops and Grapes in Davis, California (Fig. 1) and a total of 802 unique cultivars of Vitis are maintained as vines at the Plant Genetic Resources Unit in Geneva, New York (Fig. 2). Some of the cultivars in these collections are backed-up in liquid nitrogen at the National Laboratory for Genetic Resources Preservation (NLGRP) in Fort Collins, Colorado. Herein, we provide a method for cryopreserving shoot tips of Vitis that was developed at the NLGRP to preserve Vitis cultivars in liquid nitrogen.
Figure 1. Vitis in the NPGS field collection in Davis, California. Photo credit: Jean Bettoni.
Figure 2. Vitis in the NPGS field collection in Geneva, New York. Photo credit: Gayle Volk.
Vitis shoot tips can be successfully cryopreserved using several methods. One is the droplet vitrification technique (shown in Section 3.1) and the other uses a V-cryoplate (shown in Section 3.2). Both methods start with in vitro-grown Vitis plants. Nodal sections of the plants are pretreated and then shoot tips are excised and plated on Preculture Medium. They are then cryopreserved, placed into long-term storage, and then warmed and cultured for regrowth assessment.
2. Shoot tip excision
Nodal sections are cut from 2 to 3 month-old in vitro-grown Vitis plants (Fig. 3), grown on Shoot Maintenance Medium (MS + 30 g L-1 sucrose + 0.175 mg L-1 indole-3-acetic acid (IAA) + 2.5 g L-1 gellan gum at pH 5.7 (pH 6.0 prior to autoclaving)). Nodal sections are plated onto Pretreatment Medium (MS + 30 g L-1 sucrose + 0.2 mg L-1 6-benzylaminopurine (BAP) + 0.1 mM salicylic acid + 0.25 mM ascorbic acid + 0.25 mM glutathione (reduced form) + 3 g L-1 gellan gum at pH 5.7 (pH 6.0 prior to autoclaving)) in 100 x 25 mm Petri plates (50 mL of media per plate with 40 nodes each). Nodal sections are grown in a growth room at 25 oC with a 16 h photoperiod (40 μM m-2 s-1) and cultured for 2-3 weeks until the shoots are 1-2 cm high (Fig. 4). The process of preparing nodal sections is shown in Video 1.
Figure 3. Vitis stock plants used for cryopreservation. Image credit: Jean Bettoni.
Figure 4. Vitis nodal sections before (left) and after (right) two weeks of pretreatment. Image credit: Jean Bettoni.
Video 1. Preparation of Vitis nodal sections, demonstrated by Jean-Carlos Bettoni (Plant and Food New Zealand) and narrated by Remi Bonnart (NLGRP).
Apical shoot tips (1 mm) are harvested (Video 2) and plated on Preculture Medium (1/2-strength MS + 0.3 M sucrose + 0.1 mM salicylic acid + 0.25 mM ascorbic acid + 0.25 mM glutathione (reduced form) + 8 g L-1 agar at pH 5.7 (pH 6.3 prior to autoclaving)) for 3 days at 25 oC in darkness.
Video 2. Excision of Vitis shoot tips, demonstrated by Jean-Carlos Bettoni (Plant and Food New Zealand) and narrated by Remi Bonnart (NLGRP).
3.1. Shoot tip processing and liquid nitrogen exposure using droplet vitrification
Droplet vitrification is one method used for cryopreserving shoot tips. This method is particularly useful when cryoplates are not available. Shoot tips are placed into a Loading Solution, then treated with half-strength and full-strength PVS2. They are then placed onto foil strips and plunged into liquid nitrogen (LN).
Shoot tips are first removed from the Preculture Medium and placed in the Loading Solution (1/2 MS + 2 M glycerol + 0.4 M sucrose at pH 5.7 (pH 6.4 prior to autoclaving)) for 20 minutes at room temperature. They are then treated with half-strength Plant Vitrification Solution (1/2 MS + 15% (w/v) glycerol + 7.5% (w/v) ethylene glycol + 7.5% (w/v) dimethyl sulfoxide + 0.4 M sucrose at pH 5.8 (filter sterilized solution)) at room temperature for 30 minutes and then with full-strength Plant Vitrification Solution 2 (PVS2; Sakai et al., 1990) at 0 oC for 60-90 minutes (90 minutes has been the most effective time for most species/cultivars). Shoot tips are then placed into a very thin layer of PVS2 on foil strips (Fig. 5) and plunged into liquid nitrogen. This process is shown in Video 3.
Figure 5. Shoot tips in PVS2 on an aluminum foil strip. Image credit: Jean Bettoni.
Video 3. Video of the transfer of shoot tips from Vitis Preculture Medium into Loading Solution and PVS2, followed by LN exposure using the droplet-vitrification technique as demonstrated by Jean-Carlos Bettoni (Plant and Food New Zealand) and narrated by Remi Bonnart (NLGRP).
3.2. Shoot tip processing and liquid nitrogen exposure using cryoplates
V-Cryoplate cryopreservation is an alternative method to the droplet vitrification method described in Section 3.1. In this method, shoot tips are adhered to aluminum plates and are treated with Loading Solutions and PVS2, as well as LN, while attached to the cryoplate (Yamamoto et al., 2011).
Cryoplate Preparation: Place Sodium Alginate Solution (3.5-4 µL; MS (without calcium) + 0.4 M sucrose at pH 5.7 + 2 % (w/v) sodium alginate (medium viscosity-3,500 cps ; pH 6.0 prior to autoclaving)) in the wells of the aluminium plate (nº 2) using a micropipette, then place the precultured shoot tips in the wells and press the shoot tips to make them fit in the plate’s wells (Fig. 6). Pour the Calcium Chloride Solution (MS + 0.4 M sucrose + 0.1 M calcium chloride at pH 5.7 (pH 6.0 prior to autoclaving)) dropwise on the section of the aluminium plate until the cryoplate is covered (solution covers the cryoplate by surface tension) and wait for 20 minutes at room temperature to achieve polymerization.
Cryoprotectant treatments: Remove the Calcium Chloride Solution from the cryoplate with a micropipette and place cryoplate in Loading Solution (1/2 MS + 2 M glycerol + 0.4 M sucrose at pH 5.7 (pH 6.4 prior to autoclaving)) for 30 minutes at room temperature. Remove cryoplate from Loading Solution and tap it on filter paper to soak the solution into the filter paper before the addition of Plant Vitrification Solution 2 (PVS2; Sakai et al., 1990) treatment for 30-50 minutes (40 minutes has been the most effective time for the species studied) at room temperature. Remove the cryoplate from PVS2 and tap it on filter paper to soak the solution into the filter paper. Plunge the cryoplate with shoot tips into liquid nitrogen and transfer one cryoplate into each cryovial. This process is demonstrated in Video 4.
Figure 6. Shoot tips in PVS2 on a cryoplate. Image credit: Jean Bettoni.
Video 4. Video of the transfer of shoot tips from Vitis Preculture Medium into Loading Solution and PVS2, followed by LN exposure using the V-cryoplate technique as demonstrated by Jean-Carlos Bettoni (Plant and Food New Zealand) and narrated by Remi Bonnart (NLGRP).
4. Long-term storage
Aluminum foil strips or cryoplates are transferred into cryovials in a frozen state. The cryovials are loaded into each cryocane (Fig. 7), placed into labeled sleeves, and then into cryocans (Video 5). The cryocans are maintained in the liquid or vapor phase of liquid nitrogen for long-term storage within cryotanks (Fig. 8). A total of 170 shoot tips are processed per accession, with 10 shoot tips placed on each foil, and one foil placed into each cryovial. Fifteen cryovials (of 10 shoot tips each) are cryopreserved in the base collection and the shoot tips within two cryovials are warmed for viability assessments.
Figure 7. Cryovials are loaded onto cryocanes for long-term storage. Photo credit: Gayle Volk.
Video 5. Video of the transfer of cryovials into long-term storage tanks as demonstrated by Jean-Carlos Bettoni (Plant and Food New Zealand) and narrated by Remi Bonnart (NLGRP).
Figure 8. Aluminum cryoboxes in the vapor phase of liquid nitrogen. Photo credit: Gayle Volk.
5.1. Viability assessment after droplet vitrification
Shoot tips that are cryopreserved on foil strips using the droplet vitrification method can be warmed (Video 6) and assessed for regrowth (Fig. 9) according to the following procedure:
Remove cryovial from LN and place foil strip immediately in room temperature Unloading Solution (1/2 MS + 1.2 M sucrose at pH 5.7 (pH 6.6 prior to autoclaving)) and hold for 20 minutes at room temperature. Plate shoot tips onto Recovery Medium #1 (1/2-strength MS macro elements (-NH4) + MS micro elements + Vitis vitamins + 0.6 M sucrose + 8 g L-1 agar at pH 5.7 (pH 6.3 prior to autoclaving)) overnight at 25 oC in darkness. Transfer shoot tips to Recovery Medium #2 (1/2-strength MS macro elements (-NH4) + MS micro elements + Vitis vitamins + 30 g L-1 sucrose + 0.2 mg L-1 BAP + 8 g L-1 agar at pH 5.7 (pH 6.0 prior to autoclaving)) and culture for two weeks at 25 oC in darkness. Transfer shoot tips to Recovery Medium #3 (1/2-strength MS macro elements (+NH4) + MS micro elements + Vitis vitamins + 30 g L-1 sucrose + 0.2 mg L-1 BAP + 8 g L-1 agar at pH 5.7 (pH 6.0 prior to autoclaving)) and expose to light at 25 oC. Evaluate for survival/recovery after 8 weeks, transferring to fresh media if necessary.
Video 6. Video of the warming and plating process for Vitis shoot tips cryopreserved using the droplet vitrification technique as demonstrated by Jean-Carlos Bettoni (Plant and Food New Zealand) and narrated by Remi Bonnart (NLGRP).
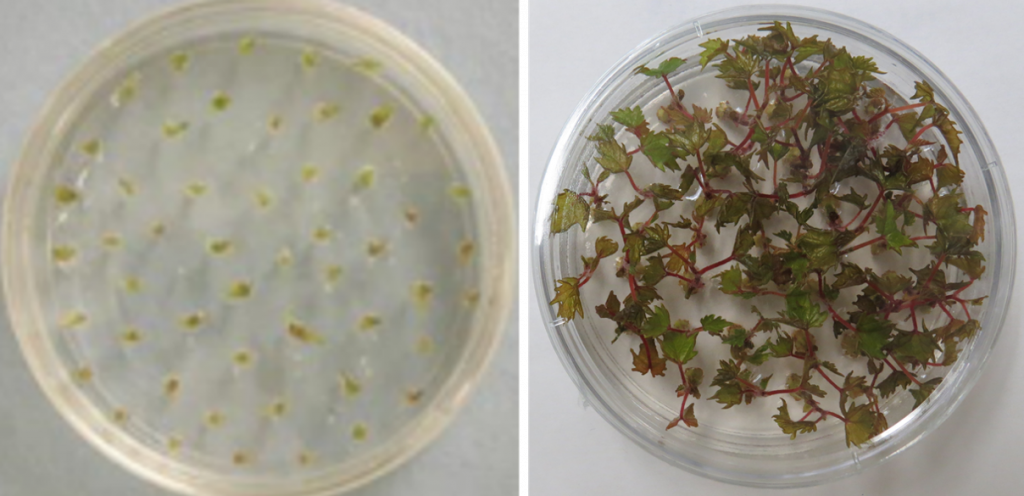
Figure 9. Vitis shoot tips recovered after droplet vitrification that have grown into viable plants. Photo credit: Jean Bettoni.
5.2. Viability assessment after V-cryoplate cryopreservation
Shoot tips that are cryopreserved on foil strips using the V-cryoplate method can be warmed (Video 7) and assessed for regrowth (Fig. 10) using the following procedure:
Remove cryovial from LN and remove the cryoplate with shoot tips from the vial. Immediately place the cryoplate into room temperature Unloading Solution, alginate beads are then detached from the cryoplates and held for 20 minutes. Plate shoot tips onto Recovery Medium #1 (1/2-strength MS macro elements (-NH4) + MS micro elements + Vitis vitamins + 0.6 M sucrose + 8 g L-1 agar at pH 5.7 (pH 6.3 prior to autoclaving)) overnight at 25 oC in darkness. Transfer shoot tips to Recovery Medium #2 (1/2-strength MS macro elements (-NH4) + MS micro elements + Vitis vitamins + 30 g L-1 sucrose + 0.2 mg L-1 BAP + 8 g L-1 agar at pH 5.7 (pH 6.0 prior to autoclaving)) and culture for two weeks at 25 oC in darkness. Transfer shoot tips to Recovery Medium #3 (1/2-strength MS macro elements (+NH4) + MS micro elements + Vitis vitamins + 30 g L-1 sucrose + 0.2 mg L-1 BAP + 8 g L-1 agar at pH 5.7 (pH 6.0 prior to autoclaving)) and expose to light at 25 oC. Evaluate for survival/recovery after 8 weeks, transferring to fresh media if necessary.
Video 7. Video of the warming and plating process for Vitis shoot tips cryopreserved using the V-cryoplate technique as demonstrated by Jean-Carlos Bettoni (Plant and Food New Zealand) and narrated by Remi Bonnart (NLGRP).
Figure 10. Vitis shoot tips recovered after V-cryoplate cryopreservation that have grown into viable plants. Photo credit: Jean Bettoni.
6. References
Bettoni JC, Bonnart R, Shepherd AN, Kretzschmar AA, Volk GM. 2019. Modifications to a Vitis shoot tip cryopreservation procedure: effect of shoot tip size and use of cryoplates. Cryoletters 40:103-112.
Bettoni JC, Pathirana R, Bonnart R, Shepherd A, Volk G. 2019. Cryopreservation of Grapevine Shoot Tips from In Vitro Plants Using Droplet Vitrification and V Cryo-plate Techniques. In: Faisal M, Alatar A, (editors). Synthetic Seeds. Springer, New York, NY. p. 469-482.
Bettoni JC, Kretzschmar AA, Bonnart R, Shepherd A, Volk GM. 2019. Cryopreservation of 12 Vitis Species Using Apical Shoot Tips Derived from Plants Grown In Vitro. HortScience 54:976-981.
Yamamoto S, Rafique T, Priyantha WS, Fukui K, Matsumoto T, Niino T. 2011. Development of a cryopreservation procedure using aluminium cryo-plates. CryoLetters 32:256-265.
Sakai A, Kobayashi S, Oiyama I. 1990. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. Var. Brasiliensis Tanaka) by vitrification. Plant Cell Reports 9:30-33.
7. Additional information
The Vitis shoot tip droplet vitrification cryopreservation procedure is available for download here.
The Vitis shoot tip regrowth procedure after droplet vitrification is available for download here.
8. Acknowledgments
Citation: Bettoni J, Bonnart R, Volk GM. 2020. Vitis Shoot Tip Cryopreservation (Droplet Vitrification and V-Cryoplate). In: Volk GM (Eds.) Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules. Fort Collins, Colorado: Colorado State University. Date accessed. Available from https://colostate.pressbooks.pub/clonalcryopreservation/chapter/vitis-shoot-tip-cryopreservation-droplet-vitrification-and-cryoplate/
This training module was made possible by:
Editors: Emma Balunek, Gayle Volk
Content providers: Jean-Carlos Bettoni, Remi Bonnart, Gayle Volk
Videographers: Mike May, Remi Bonnart
This project was funded by the USDA-ARS and in part by the National Academy of Sciences (NAS) and USAID, and any opinions, findings, conclusions, or recommendations expressed in such are those of the authors alone, and do not necessarily reflect the views of USAID or NAS. USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.