Cryopreservation of Dormant Apple Buds
Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@usda.gov
Maria Jenderek, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521.
Katheryn Chen, Department of Soil and Crop Sciences, Colorado State University, 307 University Ave., Fort Collins, Colorado 80523.
Outline
- Introduction
- Harvest dormant budwood
- Cut dormant budwood into sections
- Determine moisture content
- Desiccate and package
- Slow-cool and cryopreserve
- Long-term storage
- Warm cryopreserved budwood sections
- Graft buds onto rootstocks for viability assessment
- References
- Additional materials
- Acknowledgments
The apple dormant bud cryopreservation protocol is available for download here.
The apple dormant bud grafting protocol is available for download here.
1. INTRODUCTION
The USDA-ARS National Plant Germplasm System (NPGS) maintains more than 3000 grafted apple (Malus) accessions in a field collection in Geneva, NY (Volk et al., 2015). Dormant bud cryopreservation methods have been used to cryopreserve the NPGS apple collection since 1988 (Volk et al. 2014). A total of 2052 Malus accessions are cryopreserved at the National Laboratory for Genetic Resources Preservation (NLGRP) in Fort Collins, Colorado (Volk et al. 2014). First, dormant twigs are harvested from field-grown trees and then shipped to the NLGRP. The twigs are then cut into nodal sections and dried to a moisture content of 25-30% (fresh weight basis, fwb). They are then packaged into polyolefin tubes and cooled at a rate of 1 oC hr-1 to -30 oC and held overnight. Then the tubes containing dormant buds are plunged into the vapor phase of liquid nitrogen and placed into cryotanks for long-term storage. Buds are warmed to 2 to 5 oC and then placed into moist peat moss for 14 to 21 days. They are then grafted onto rootstocks for viability assessment and plant recovery. The cryopreservation and regrowth assessment methods are described below.
2. Harvest dormant budwood
Dormant budwood twigs are harvested from the previous season’s growth of field-grown apple trees (Fig. 1; Video 1). In the USDA, budwood is derived from trees grown in Geneva, NY in January, ideally after temperatures have been below 0 oC for a few days. The harvested twigs are 5-7 mm in diameter, have about 10 nodal sections each, and should total 17-20 twigs (170-200 nodal sections) per accession; appropriate budwood is shown in Figure 2, in contrast to the material shown in Figure 3. The budwood is packaged in bundles and sent by overnight courier to NLGRP. It is kept at -5 oC in sealed plastic bags for up to a few weeks until it is used for cryopreservation.
Figure 1. Dormant budwood is harvested from field-grown apple trees. Photo credit: Reece Perrin.
Video 1. Video of Reece Perrin (Cornell University) explaining the process of harvesting apple dormant budwood for distribution or use in cryopreservation.
Figure 2. Example of apple dormant budwood that will be cut into nodal sections and cryopreserved. Photo credit: Gayle Volk.
Figure 3. Example of dormant budwood that has thin spur branches that will not be used for cryopreservation. Photo credit: Gayle Volk.
3. Cut dormant budwoods into sections
Dormant budwood is cut into 35 mm long sections with a single node centered on the segment (Fig. 4). The terminal bud on the branch is discarded. For mass-processing, budwood is cut using a bandsaw (Fig. 5) with the appropriate safety precautions. This process is shown in Video 2.
Figure 4. A single-node apple dormant budwood section. Photo credit: Gayle Volk.
Figure 5. Image of a bandsaw being used to cut 35 mm sections of dormant budwood. Photo credit: Gayle Volk.
Video 2. Technician Katheryn Chen (Colorado State University) demonstrating the process of cutting 35 mm apple dormant budwood sections that will be cryopreserved.
4. Determine initial moisture content
The budwood moisture content must be reduced from field moisture contents (43-53%) to 25-30% moisture content (fwb) for each accession that will be cryopreserved (Jenderek et al., 2011). First, the initial moisture content of 10 nodal sections is calculated by measuring the fresh weight and dry weight. Dry weight is taken after sections, placed in an oven-safe tray (Fig. 6) are dried for three days in an oven at 100 oC. Weighing and drying is shown in Video 3. Once weights are established, the following equation is used to calculate the initial moisture content: (FW-DW)/FW x 100%. The total fresh weight of the remaining nodal sections is measured and the desired ending weight is calculated based on a desired moisture content of 25-30% (fwb).
Figure 6. Ten nodal sections, each containing a dormant bud, are each weighed and placed into vials. They are dried at 100 oC for 3 days and then weighed again so that the initial moisture content of the nodal sections can be calculated. Photo credit: Gayle Volk.
Video 3.Technician Katheryn Chen weighs the nodal sections as part of the process of calculating the original moisture content, and then places them into an oven set at 100 oC for 3 days to determine the nodal section dry weight.
5. Desiccate and package
The remaining original nodal sections are weighed and placed into plastic net-lined trays (Fig. 7) and desiccated in a chamber at -5 oC and 35% relative humidity (Video 4). The buds are re-weighed every 1 to 7 days to determine if the target moisture content of 25 to 30% has been achieved.
Figure 7. Dormant buds on a net-lined plastic tray, ready for desiccation at -5 oC. Photo credit: Gayle Volk.
Video 4. Dormant buds are weighed using an electronic balance and the weight data are captured directly into a spreadsheet. These are placed into a chamber at -5 oC and 35% relative humidity to reduce the moisture content to 25-30% (fwb).
When the nodal sections are predicted to have 25-30% moisture content, they are placed into polyolefin tubes (Fig. 8) with 10 nodal sections per tube. The diameter of the selected polyolefin tube is dependent upon the thickness of the nodal sections. A total of 17 polyolefin tubes are prepared for each accession, material permitting. The tubes are heat-sealed. The tubes of dormant buds are labeled with the accession number, NSSL inventory number, taxon, cultivar name, bar code and date (Fig. 9). They are then placed into aluminum cryoboxes (Fig 10) for slow-cooling. The packaging process is shown in Video 5.
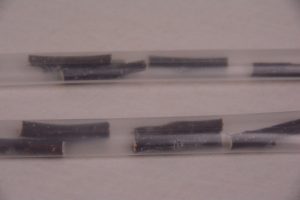
Figure 8. Nodal sections inside polyolefin tubes. Photo credit: Gayle Volk.
Figure 9. A polyolefin tube with nodal sections containing dormant buds ready for cryoprocessing. Photo credit: Gayle Volk.
Figure 10. Polyolefin tubes of apple nodal sections in a cryobox. Photo credit: Maria Jenderek.
Video 5. Technician Katheryn Chen fills and seals polyolefin tubes with 10 nodal sections each.
6. Slow-cool and cryopreserve
The boxes are placed into a programmable cooler and are cooled from -5 oC to -30 oC at a rate of 1 oC per hour, and are then held at -30 oC for 24 hours (Video 6). The cryoboxes are then transferred to the vapor phase of liquid nitrogen.
Video 6. Technician Katheryn Chen programs the cooler that is used to reduce the temperature of the dormant buds from -5 oC to -30 oC.
7. Long-term storage
The transfer tank with the cryoboxes containing the tubes of nodal sections is taken to the NLGRP vault where the long-term storage tanks are located (Video 7). Each cryobox is transferred to a specific location within the cryotanks that is recorded in the GRIN-Global database. An accession is considered securely backed-up when the viability levels are at least 40% and there are at least 60 nodal sections that are predicted to have viable dormant buds, based on grafting assessments.
Video 7. Technician Katheryn Chen transfers the cryobox containing the tubes of nodal sections to the vapor phase of liquid nitrogen and takes it to the NLGRP storage vault for long-term storage.
8. Warm cryopreserved budwood sections
One or two polyolefin tubes containing nodal sections with cryopreserved apple buds are retrieved from the cryotanks for viability assessments (Video 8; Fig. 11). These tubes are transferred to 2 oC and warmed for 24 hours (Fig. 12). Either 10 or 20 nodal sections will be warmed and grafted.
Video 8. Technician Katheryn Chen retrieving a tube of dormant buds from the cryotank for viability assessment.
Figure 11. Image of technician Katheryn Chen retrieving tubes of dormant apple nodal sections for viability assessments. Photo credit: Gayle Volk.
Figure 12. Image of warmed tubes ready to be shipped to Geneva, NY for grafting and viability assessment. Photo credit: Katheryn Chen.
9. Graft buds onto rootstocks for viability assessment
Bare-root apple seedling rootstocks (one growing season old) are purchased from a nursery (Fig. 13). They are potted into cone-tainers that are a little less than a quart-size (Video 9). The potted rootstocks are grown in the greenhouse for 3-4 weeks before budding. The rootstocks should be actively growing and leafed out nicely.
Figure 13. Bare-root rootstocks are purchased from a nursery, planted in cone-tainers, and then grown in the greenhouse. Photo credit: John Keeton.
Video 9. Reece Perrin (Cornell University) unpacks the bare-root rootstocks and prunes the roots.
Cryopreserved nodal sections are shipped by overnight courier to Geneva, NY. They are placed into moist peat moss and allowed to rehydrate at 2-4 oC for 14-21 days (Video 10). Then they are patch-budded onto the seedling rootstocks with two cryopreserved buds on each seedling rootstock (Video 11).
Video 10. John Keeton (Cornell University) demonstrates how nodal sections are rehydrated.
Video 11. John Keeton demonstrates the apple bud grafting procedure. Note: this video uses a bare-root plant and field-source dormant buds to demonstrate grafting. Trees grafted for cryopreservation viability assessments have been grown in the greenhouse for 3 weeks prior to grafting and buds are cut from the cryopreserved nodal sections that have been rehydrated.
After three weeks, the budding rubbers are removed and the tops of the rootstocks cut off above the grafted buds (Video 12; Fig. 14). Viability data are collected after 8 to 10 weeks on each of the two buds per rootstock. Buds are scored as alive if they are pushing out, growing, green, and forming a shoot (Fig. 15, 16).
The viability data for each grafted accession are added to the GRIN-Global database. Accessions are considered to be successfully cryopreserved if there are at least 40% viable buds predicted to be in each polyolefin tube (4 out of 10 bud sections have viable buds) and if there are at least 60 buds predicted to be viable in LN for the accession.
Video 12. John Keeton demonstrates the process of pruning off the top of the rootstock above the graft union 21 days after grafting.
Figure 14. Grafted trees are topped (pruned above the graft union) 21 days after grafting. Photo credit: John Keeton.
Figure 15. Image of a cryopreserved apple dormant bud that formed a shoot after it was grafted onto a rootstock. Photo credit: Remi Bonnart.
Figure 16. A close-up image of a cryopreserved apple dormant bud that formed a shoot after it was grafted onto a rootstock. Photo credit: Remi Bonnart.
10. References
Forsline PL, Towill L, Waddell J, Stushnoff C, Lamboy W, McFerson JR. 1998. Recovery and longevity of cryopreserved dormant apple buds. J. Amer. Soc. Hort. Sci. 123(3):365-370.
Jenderek MM, Forsline P, Postman J, Stover E, Ellis D. 2011. Effect of geographical location, year, and cultivar on survival of Malus sp. dormant buds stored in vapors of liquid nitrogen. HortScience 46:1230-1234.
Towill LE, Forsline PL, Walters C, Waddell J, Laufmann J. 2004. Cryopreservation of Malus germplasm using a winter vegetative bud method: Results from 1915 accessions. CryoLetters 25(5):323-334.
Volk GM, Chao CT, Norelli J, Brown SK, Fazio G, Peace C, McFerson J, Zhong G-Y, Bretting P. 2015. The vulnerability of US apple (Malus) genetic resources. Genet. Resour. Crop Evol. 62:765-794.
Volk GM, Jenderek M, Chao CT. 2014. Prioritization of Malus accessions for collection cryopreservation at the USDA-ARS National Center for Genetic Resources Preservation. Acta Hort. 112:267-272.
Volk GM, Waddell J, Bonnart R, Towill L, Ellis D, Luffman M. 2008. High viability of dormant Malus buds after 10 years of storage in liquid nitrogen vapour. CryoLetters 29(2)89-95.
11. Additional materials
The apple dormant bud cryopreservation protocol is available for download here.
The apple dormant bud grafting protocol is available for download here.
12. Acknowledgments
Citation: Volk GM, Jenderek M, Chen K. 2020. Cryopreservation of Dormant Apple Buds. In: Volk GM (Eds.) Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules. Fort Collins, Colorado: Colorado State University. Date accessed. Available from https://colostate.pressbooks.pub/clonalcryopreservation/chapter/apple-dormant-bud-cryopreservation/
This training module was made possible by:
Editors: Emma Balunek, Gayle Volk, Katheryn Chen
Content providers: Maria Jenderek, John Keeton, Katheryn Chen, Gayle Volk
Videographers: Mike May, Martin Hooker, Emma Balunek, Gayle Volk
USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.