Citrus Shoot Tip Cryopreservation

Gayle M. Volk, USDA-ARS National Laboratory for Genetic Resources Preservation (retired), 1111 S. Mason St., Fort Collins, Colorado 80521. Gayle.Volk@colostate.edu
Robert Krueger, USDA-ARS National Clonal Repository for Citrus and Dates, 1060 Martin Luther King Blvd., Riverside, California 92507.
Brittany Moreland, USDA-ARS National Clonal Repository for Citrus and Dates, 1060 Martin Luther King Blvd., Riverside, California 92507.
Remi Bonnart, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521.
Ashley Shepherd, USDA-ARS National Laboratory for Genetic Resources Preservation, 1111 S. Mason St., Fort Collins, Colorado 80521.
Outline
- Introduction to the USDA National Plant Germplasm System Citrus collection
- Introduction to the citrus shoot tip cryopreservation procedure
- Harvesting and preparing budwood
- Cutting nodal sections of budwood
- Surface sterilization
- Shoot tip excision
- Shoot tip processing and liquid nitrogen exposure
- Long-term storage
- References
- Additional information
- Acknowledgments
The citrus shoot tip cryopreservation procedure is available to download here.
1. Introduction to the USDA National Plant Germplasm System Citrus Collection
Access to diverse citrus genetic resources is critical for breeding new citrus cultivars that yield more and have higher quality fruit with improved resistance to pathogens and to changing environmental conditions. The USDA-ARS National Plant Germplasm System (NPGS) maintains a very diverse collection of about 600 pathogen-tested accessions of citrus under protected screenhouse conditions at the National Clonal Germplasm Repository for Citrus and Dates (NCGRCD) in Riverside, California (Video 1). Additional non-pathogen-tested accessions are maintained in cooperation with the University of California, Riverside. Many of the accessions within the NPGS citrus collection are disease-free and available to scientists worldwide for citrus research and for the development of new cultivars. The collection is backed-up in greenhouses at the University of California, Riverside, but is still somewhat susceptible to extreme weather, pests, pathogens, vandalism, etc. because trees are kept in pots within vulnerable greenhouses and screenhouses. Consequently, the pathogen-tested citrus collection is being cryopreserved for long-term security back-up at the National Laboratory for Genetic Resources Preservation (NLGRP) in Fort Collins, Colorado.
This chapter describes the process that enables cryopreservation of citrus shoot tips.
Video 1. Robert Krueger, curator of the NPGS citrus collection, provides a video-introduction to the collection.
2. Introduction to the citrus shoot tip cryopreservation procedure
The citrus shoot tip cryopreservation procedure has many steps that will be described and demonstrated. It starts with the harvest of budwood from screenhouse plants in the USDA National Plant Germplasm System genebank in Riverside, California. These cuttings are then shipped to the NLGRP in Fort Collins, Colorado to be cryopreserved. After receipt, the budwood is cut into sections, surface sterilized, and then shoot tips are excised. Shoot tips are then pre-treated and then immersed in cryoprotectant solutions. They are mounted on foil strips and plunged into liquid nitrogen. The strips are placed into cryovials and transferred to liquid nitrogen tanks for long-term preservation. To assess regrowth, a subset of the cryopreserved shoot tips are warmed and micrografted onto in vitro seedling rootstocks (see “Citrus micrografting for regrowth after shoot tip cryopreservation“).
3. Harvesting and preparing budwood
Budwood is collected from the pathogen-tested screenhouse collection at the NCGRCD in Riverside, California for long-term preservation at NLGRP (Video 2). The citrus trees in pots at the NCGRCD are pruned in March each year. The new flushes of growth that will be cryopreserved are harvested (Fig. 1) between the following September and January. Older growth (Fig. 2) should be avoided. In February, the floral buds emerge (Fig. 3) and the harvest season for cryopreservation is over. The vegetative budwood is about 3-5 mm in diameter. Leaves and petioles are removed from the budwood.
Video 2. Brittany Moreland, Biological Science Technician at NCGRCD, describes the citrus budwood harvesting process in this video.
Figure 1. Citrus budwood at the correct stage for harvest. Photo credit: Gayle Volk.
Figure 2. Citrus budwood that is too old for use in cryopreservation. Photo credit: Gayle Volk.
Figure 3. Citrus budwood with buds that have started to grow and is not acceptable for use in cryopreservation. Photo credit: Gayle Volk.
After harvest, the budwood is cleaned, treated, and then inspected prior to shipment to NLGRP. The budwood is rinsed with a mild detergent solution in ambient temperature tap water with a 0.5% solution of sodium hypochlorite, and rinsed thoroughly with water again (Video 3). The budwood is then dipped for 3 minutes in a solution containing a fungicide, insecticide, and miticide. Fenpropathrin (Danitol) is currently used, with a copper fungicide (Kop-R-Spray), both at a rate of 1.25 mL L-1 of ambient temperature water. The ends of the budsticks can be dipped in melted paraffin wax to help reduce desiccation.
Video 3. Video of citrus budwood being treated with sodium hypochlorite and then rinsed.
The box filled with budwood is shipped overnight by courier to NLGRP and the materials arrive with the phytosanitary certificate, certificate of inspection, and packing list (Video 4). It is best to use the budwood within 2-3 days of arrival; however, citrus budwood can be stored in sealed plastic bags for up to four weeks at 4 oC before being used for shoot tip cryopreservation.
Video 4. Silent video showing a box of citrus budwood that has arrived at NLGRP. The paperwork and twigs are inside.
4. Cutting nodal sections of budwood
Budwood (Fig. 4) must be cut into one- or two-node sections using pruners prior to surface sterilization. Nodal sections are placed into water containing a drop of Tween 20 (1 drop per 100 mL water), as seen in Figure 5 and Video 5.
Figure 4. Budwood that has been harvested and shipped to NLGRP for cryopreservation. Photo credit: Remi Bonnart.
Figure 5. Nodal sections of budwood that will be surface sterilized. Photo credit: Remi Bonnart.
Video 5. Silent video demonstrating the cutting of nodal sections.
5. Surface sterilization
Nodal sections of budwood are surface sterilized. A mesh screen is placed over the flask and the nodal sections are rinsed 3 times with tap water. They are disinfected with 70% isopropanol for 2 minutes and then rinsed three times with tap water (Video 6).
Video 6. Silent video showing the 70% isopropanol treatment and rinsing of the nodal sections.
The flask with the nodal sections is then placed into a laminar flow hood. The nodal sections are treated with 10% commercial bleach (0.825% sodium hypchlorite final concentration) and a drop of Tween 20 (1 drop per 100 mL water) for 10 minutes, followed by three rinses with sterile distilled water (Video 7).
Video 7. Silent video showing the sodium hypochlorite treatment and rinsing of the nodal sections.
6. Shoot tip excision
Shoot tips (1-1.5 mm) are excised from each nodal section using a dissecting microscope in a laminar flow hood (Video 8). Figure 6 shows the citrus nodal section prior to excision, and Figures 7 and 8 show examples of excised shoot tips. All instruments are sterilized before use. If needed, plant materials can be dipped in a Shoot Tip Dip/Rinse Solution to prevent dehydration. Shoot tips are placed in a Liquid Preculture Medium in a small Petri dish (35 x 10 mm) in darkness at 25 oC overnight before undergoing the cryopreservation process the next day.
Video 8. Video of shoot tip excision process from the surface-sterilized nodal sections. Excision performed and narrated by Remi Bonnart, Biological Science Technician at NLGRP.
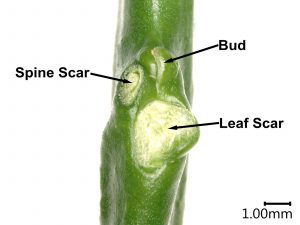
Figure 6. Diagram of a citrus budwood node, showing the leaf scar, spine scar, and bud that contains the shoot tip. Photo credit: Remi Bonnart.
Figure 7. Example 1. Excised shoot tip that contains the meristem, several leaf primordia, and basal tissue. Photo credit: Remi Bonnart.
Figure 8. Example 2. Excised shoot tip that contains the meristem, several leaf primordia, and basal tissue. Photo credit: Remi Bonnart.
7. Shoot tip processing and liquid nitrogen exposure
Excised shoot tips are treated with cryoprotectant solutions, loaded onto aluminum foil strips and plunged into liquid nitrogen. The foil strips are then placed into cryovials and prepared for long-term storage. The steps are described below and shown in Video 9.
Video 9. Remi Bonnart (NLGRP) demonstrates the shoot tip processing and cryopreservation process.
1.8 mL cryovials (Fig. 9) are labeled with identifying information, including Plant identifier number (PI), NSSL inventory number, genus/species, cultivar name, date, and technician initials. Labels tolerate LN exposure and storage and completely wrap around the tube.
Figure 9. Cryovial labeled with identifying information. Photo credit: Remi Bonnart.
The Petri dish with shoot tips is taken from the overnight incubation conditions and it is placed into the laminar flow hood. The incubation solution is first removed and replaced with Loading Solution (2 M glycerol + 0.4 M sucrose + 1/2 strength MS solution (pH 5.8)); and shoot tips are incubated at room temperature for 20 minutes. The Loading Solution is then removed and replaced with ice-cold Plant Vitrification Solution (PVS2) (Sakai et al. 1990) and shoot tips are incubated on ice in PVS2 for 30-45 minutes. During the last 5 minutes of the incubation, an elongated “pool” of PVS2 is added to each sterile aluminum foil strips (0.5 cm wide x 2-3 cm long) and 10 shoot tips are transferred into the PVS2 on the foil strip. The strips are then plunged into liquid nitrogen in a small dewar. Cryovials are cooled in the same liquid nitrogen. Liquid nitrogen is removed from the vial and one foil with shoot tips is loaded into each cryovial (Fig. 10). The lids are placed on the cryovials and the cryovials are then ready to be prepared for long-term storage.
Figure 10. Cryopreserved shoot tips on foil strips are transferred to cryovials for long-term storage. Photo credit: Peggy Greb.
8. Long-term storage
Cryovials containing aluminum foil strips are held in a dewar of liquid nitrogen until they are loaded onto cryocanes, 5 vials per cane. The canes are then placed into cryoboxes for long-term storage in liquid nitrogen vapor within cryotanks. This process descibed below and shown in Video 10.
Video 10. Cryovials are transferred to cryocanes and loaded into cryoboxes for long-term storage in the vapor phase of LN.
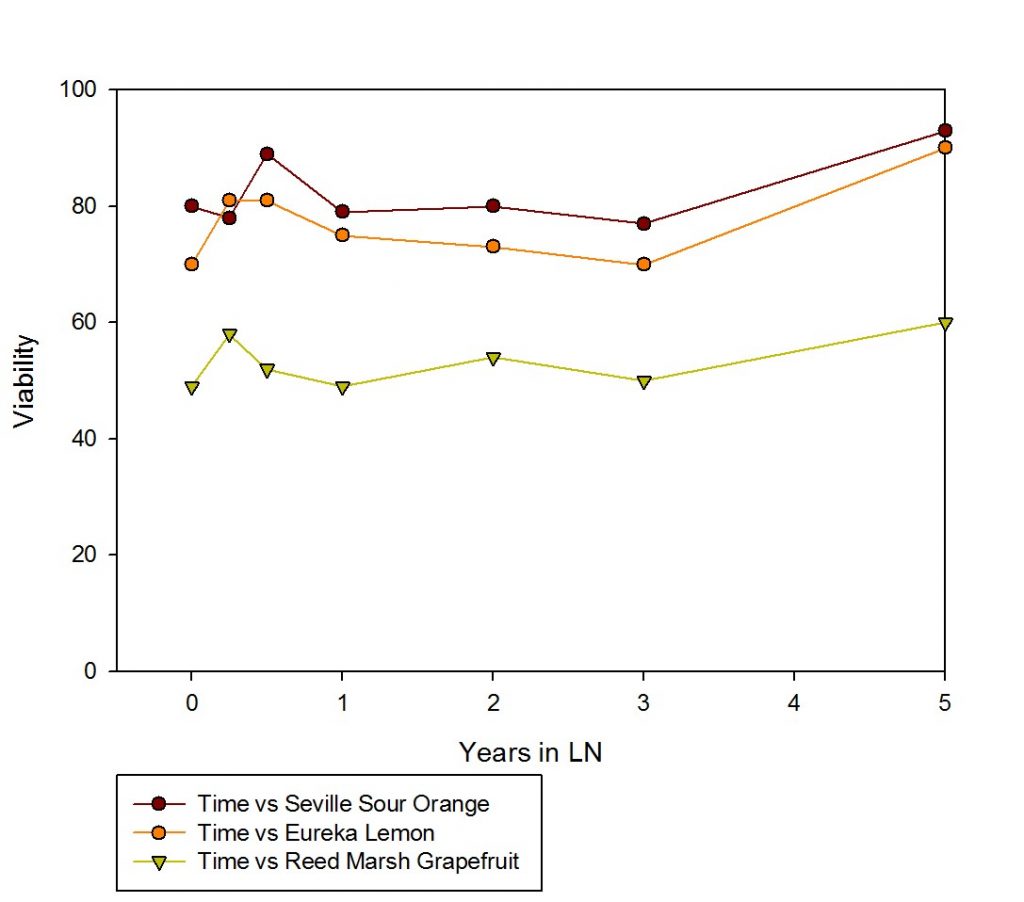
The standard procedure for citrus cryopreservation at NLGRP is to excise and cryopreserve 170 shoot tips, with 10 per foil strip and one foil strip per cryovial. Five cryovials are placed on each cryocane (Fig. 11), and the three canes (with 5 cryovials each) are placed into separate cryoboxes. The boxes can then be put into different cryotanks (Fig. 12) for increased security and safekeeping. The remaining two cryovials (each containing 10 shoot tips mounted on a foil strip) are kept in a storage box in the vapor phase of liquid nitrogen for use in viability assessments. One cryovial is warmed and the viability will be assessed for the 10 shoot tips (see “Citrus micrografting for regrowth after shoot tip cryopreservation“). The second vial is available if a second viability assessment is needed. If the viability of the 10 shoot tips is 40% or greater, the accession is considered to be successfully cryopreserved. These are documented in GRIN-Global and transferred into NLGRP cryotanks (Fig. 13). Storage in secure LN vapor tanks ensures the citrus germplasm is maintained long-term (Fig. 14).
Figure 11. Cryovials are loaded onto cryocanes while submerged in LN. Photo credit: Gayle Volk.
Figure 12. Cryopreserved citrus is stored in cryoboxes in the vapor phase of LN. Photo credit: Gayle Volk.
Figure 13. Cryotanks for long-term storage at NLGRP. Photo credit: Gayle Volk.
Figure 14. This figure shows the regrowth levels of three citrus cultivars after up to five years of storage in liquid nitrogen.
Viability assessments are made using a micrografting procedure. Shoot tip warming and micrografting is demonstrated in the next chapter titled “Citrus micrografting for regrowth after shoot tip cryopreservation“.
9. References
Sakai A, Kobayashi S, Oiyama I. 1990. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. Var. Brasiliensis Tanaka) by vitrification. Plant Cell Reports 9:30-33.
Volk GM, Bonnart R, Krueger R, Lee R. 2012. Cryopreservation of citrus shoot tips using micrografting for recovery. CryoLetters 33:418-426.
Volk GM, Bonnart R, Shepherd A, Krueger R, Lee R. 2015. Cryopreservation of citrus for long-term conservation. Acta Hort. 1065:187-192.
Volk GM, Bonnart R, Shepherd A, Yin Z, Lee R, Polek ML, Krueger R. 2016. Citrus cryopreservation: Viability of diverse taxa and histological observations. Plant Cell Tissue and Organ Culture 128:327-334. DOI 10.1007/s11240-016-1112-4
Volk GM, Jenderek MM, Walters C, Bonnart R, Shepherd A, Skogerboe D, Hall BD, Moreland B, Krueger R, Polek ML. 2019. Implementation of Citrus shoot tip cryopreservation in the USDA-ARS National Plant Germplasm System. Acta Hort. 1234: 329-334.
10. Associated information
The citrus shoot tip cryopreservation procedure is available to download here.
A large-scale citrus cryopreservation effort took place at NLGRP in 2016-2017. Reports were provided on the monthly progress of the project. A compilation of the monthly reports is available here.
11. Acknowledgments
Citation: Volk GM, Krueger R, Moreland B, Bonnart R, Shepherd A. 2020. Citrus Shoot Tip Cryopreservation. In: Volk GM (Eds.) Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules. Fort Collins, Colorado: Colorado State University. Date accessed. Available from https://colostate.pressbooks.pub/clonalcryopreservation/chapter/citrus-cryopreservation/
This training module was made possible by:
Editors: Emma Balunek, Gayle Volk
Content providers: Robert Krueger, Brittany Moreland, Remi Bonnart
Videographers: Mike May, Remi Bonnart, Gayle Volk
USDA is an equal opportunity provider, employer, and lender. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.